chapter 3 notes for power point
... as moving waves that have given frequencies, speeds, and wavelengths. • In empty space, light waves travel at 2.998 108 m/s. • The wavelength is the distance between two consecutive peaks or troughs of a wave. • The distance of a wavelength is usually measured in meters. • The electromagnetic spec ...
... as moving waves that have given frequencies, speeds, and wavelengths. • In empty space, light waves travel at 2.998 108 m/s. • The wavelength is the distance between two consecutive peaks or troughs of a wave. • The distance of a wavelength is usually measured in meters. • The electromagnetic spec ...
(null): 110.ReactionsIntro
... b) KE transformed into chem PE 3) Do reduced version of Zn & HCl: one zinc pellet in test tube plus a few ml of HCl. While bubbling discuss where energy is stored and where it goes 4) Return to reaction, have Ss feel test tube (warm!) & decide if reaction followed Option 1 or 2 d. “Re-arrange” colli ...
... b) KE transformed into chem PE 3) Do reduced version of Zn & HCl: one zinc pellet in test tube plus a few ml of HCl. While bubbling discuss where energy is stored and where it goes 4) Return to reaction, have Ss feel test tube (warm!) & decide if reaction followed Option 1 or 2 d. “Re-arrange” colli ...
ramsauer - UT Relativity Group
... the potential. If the Ramsauer-Townsend effect was not occurring then one would expect the two graphs to be approximately the same, however it is clear that the graph of the plate current at room temperature deviates tremendously due to the effect. (see graph I(p*) and I(p) v.s. root Va next page). ...
... the potential. If the Ramsauer-Townsend effect was not occurring then one would expect the two graphs to be approximately the same, however it is clear that the graph of the plate current at room temperature deviates tremendously due to the effect. (see graph I(p*) and I(p) v.s. root Va next page). ...
PHYSICAL SETTING CHEMISTRY
... 37 Which general trends in first ionization energy and electronegativity values are demonstrated by Group 15 elements as they are considered in order from top to bottom? (1) The first ionization energy decreases and the electronegativity decreases. (2) The first ionization energy increases and the ...
... 37 Which general trends in first ionization energy and electronegativity values are demonstrated by Group 15 elements as they are considered in order from top to bottom? (1) The first ionization energy decreases and the electronegativity decreases. (2) The first ionization energy increases and the ...
Quantum Numbers, Orbitals, and Probability Patterns
... come from Schrödinger’s equation, and the fourth one comes from an extension of the theory.) These four numbers completely describe the energy of an electron. Each electron has exactly four quantum numbers, and no two electrons have the same four numbers. The statement that no two electrons can have ...
... come from Schrödinger’s equation, and the fourth one comes from an extension of the theory.) These four numbers completely describe the energy of an electron. Each electron has exactly four quantum numbers, and no two electrons have the same four numbers. The statement that no two electrons can have ...
Time-Dependent Electron Interactions in Double
... of WP2 and, accordingly, the radial distance from the ion and the time at which the wave packets first overlap [6,7]. The colinearly propagating, vertically polarized lasers are focused into the Ba beam between two parallel field plates in a time-of-flight spectrometer. Approximately 50 ns after the ...
... of WP2 and, accordingly, the radial distance from the ion and the time at which the wave packets first overlap [6,7]. The colinearly propagating, vertically polarized lasers are focused into the Ba beam between two parallel field plates in a time-of-flight spectrometer. Approximately 50 ns after the ...
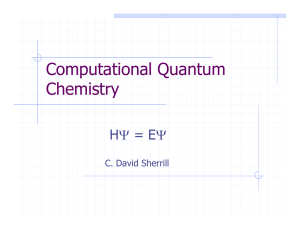
Computational Quantum Chemistry
... Chemistry (Kohn and Pople) recognized work in this area. Use the density instead of complicated manyelectron wavefunctions. Basic idea: minimize the energy with respect to the density. Relationship of energy to density is the “functional” E[ρ] (true form of this functional is unknown: use approx.) ...
... Chemistry (Kohn and Pople) recognized work in this area. Use the density instead of complicated manyelectron wavefunctions. Basic idea: minimize the energy with respect to the density. Relationship of energy to density is the “functional” E[ρ] (true form of this functional is unknown: use approx.) ...
Atomic Theory and Periodicity Questions
... (b) In terms of these configurations, explain why the values of the first and second ionization energies of Mg are significantly lower than the values for Ar, whereas the third ionization energy of Mg is much larger than the third ionization energy of Ar. (c) If a sample of Ar in one container and a ...
... (b) In terms of these configurations, explain why the values of the first and second ionization energies of Mg are significantly lower than the values for Ar, whereas the third ionization energy of Mg is much larger than the third ionization energy of Ar. (c) If a sample of Ar in one container and a ...
File
... When comparing enthalpy changes for formation reactions of different compounds, we must choose a reference energy state. It is convenient to set the enthalpies of elements in their most stable form at SATP to be zero. As an arbitrary convention, for the sake of simplicity, all other enthalpies of ...
... When comparing enthalpy changes for formation reactions of different compounds, we must choose a reference energy state. It is convenient to set the enthalpies of elements in their most stable form at SATP to be zero. As an arbitrary convention, for the sake of simplicity, all other enthalpies of ...
two electron energy sprectrum in concentrical quantum ribbons
... single semiconductor nanotube [5]. The results show fundamental differences related to electrical current distributions generated in cylindrical and planar structures. Despite the importance of this study [5], we consider also interesting to analyze the case of two particles constrained to move into ...
... single semiconductor nanotube [5]. The results show fundamental differences related to electrical current distributions generated in cylindrical and planar structures. Despite the importance of this study [5], we consider also interesting to analyze the case of two particles constrained to move into ...
SOLID-STATE PHYSICS II 2007 O. Entin-Wohlman
... ∗ ∗ ∗ exercise: Prepare a similar table for the ions with partially filled f −shell (L = 3). Hund’s three rules determine the ground state(s) of the partially-filled ion. However, that ground state is still degenerate. Take for example, the case n = 2 in the Table. After applying Hund’s first and se ...
... ∗ ∗ ∗ exercise: Prepare a similar table for the ions with partially filled f −shell (L = 3). Hund’s three rules determine the ground state(s) of the partially-filled ion. However, that ground state is still degenerate. Take for example, the case n = 2 in the Table. After applying Hund’s first and se ...
Unit 2 – Electrons and Periodic Behavior Cartoon courtesy of
... Each electron in an atom has a unique set of 4 quantum numbers which describe it. Principal quantum number Angular momentum (orbital) quantum ...
... Each electron in an atom has a unique set of 4 quantum numbers which describe it. Principal quantum number Angular momentum (orbital) quantum ...
15.2 Electrons and Chemical Bonds
... The discovery of energy levels in the atom solved a 2,000-year-old mystery. The mystery was why elements combined with other elements only in particular ratios (or not at all). For example, why do two hydrogen atoms bond with one oxygen atom to make water? Why isn’t there a molecule with three (H3O) ...
... The discovery of energy levels in the atom solved a 2,000-year-old mystery. The mystery was why elements combined with other elements only in particular ratios (or not at all). For example, why do two hydrogen atoms bond with one oxygen atom to make water? Why isn’t there a molecule with three (H3O) ...
Practice exam - Dynamic Science
... 30) Chemists react organic acids with alcohols to form: a) proteins; b) esters; c) hydrogen gas; d) carbon dioxide. 31) Which one of the following is a renewable energy source? a) Natural gas b) Ethanol c) Uranium d) all of the above 32) Which of the following are carbon neutral fuels? a) Ethanol b) ...
... 30) Chemists react organic acids with alcohols to form: a) proteins; b) esters; c) hydrogen gas; d) carbon dioxide. 31) Which one of the following is a renewable energy source? a) Natural gas b) Ethanol c) Uranium d) all of the above 32) Which of the following are carbon neutral fuels? a) Ethanol b) ...
Quiz #5: Physics 203
... This cannot be solved analytically. You will have to solve it numerically. Different values of u will give you different solutions (and different numbers of solutions). You can use a graphing calculator, Maple, or a program such as Excel (look for functions with names like “Solver” or “Goal Seek”). ...
... This cannot be solved analytically. You will have to solve it numerically. Different values of u will give you different solutions (and different numbers of solutions). You can use a graphing calculator, Maple, or a program such as Excel (look for functions with names like “Solver” or “Goal Seek”). ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.