Holt Modern Chemistry Workbook: intro - ch 5
... example, a piece of coal keeps its size and its shape, regardless of the container it is in. Particles in solids are packed together. The particles can vibrate back and forth, but they cannot change position. The particles are held together because they are attracted to each other. Liquids A liquid ...
... example, a piece of coal keeps its size and its shape, regardless of the container it is in. Particles in solids are packed together. The particles can vibrate back and forth, but they cannot change position. The particles are held together because they are attracted to each other. Liquids A liquid ...
Type - Enrico Fermi High
... points increase. Account for this based on IMF’s. As go down, MM goes up, so dispersion forces go up, so VP down and BP up. Why does water have an unusually high boiling point? Water forms lots of hydrogen bonds with itself. ...
... points increase. Account for this based on IMF’s. As go down, MM goes up, so dispersion forces go up, so VP down and BP up. Why does water have an unusually high boiling point? Water forms lots of hydrogen bonds with itself. ...
Stoichiometry: Calculations with Chemical Formulas and
... (a) The left box, which represents reactants, contains two kinds of molecules, those composed of two oxygen atoms (O2) and those composed of one nitrogen atom and one oxygen atom (NO). The right box, which represents products, contains only molecules composed of one nitrogen atom and two oxygen atom ...
... (a) The left box, which represents reactants, contains two kinds of molecules, those composed of two oxygen atoms (O2) and those composed of one nitrogen atom and one oxygen atom (NO). The right box, which represents products, contains only molecules composed of one nitrogen atom and two oxygen atom ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... 1. It is said that a reaction “takes place” or “is spontaneous” or “goes to completion” only if one of the products is water, a gas, or a precipitate. 2. For metathesis reactions, if the products formed are also aqueous, we say the reaction is “not spontaneous” or doesn’t go to competition. Aqueous ...
... 1. It is said that a reaction “takes place” or “is spontaneous” or “goes to completion” only if one of the products is water, a gas, or a precipitate. 2. For metathesis reactions, if the products formed are also aqueous, we say the reaction is “not spontaneous” or doesn’t go to competition. Aqueous ...
BRIEF ANSWERS TO SELECTED PROBLEMS APPENDIX G
... conservation: the total quantity of matter does not change. (c) Law of multiple proportions: two elements can combine to form two different compounds that have different proportions of those elements. 2.16(a) No, the percent by mass of each element in a compound is fixed. (b) Yes, the mass of each e ...
... conservation: the total quantity of matter does not change. (c) Law of multiple proportions: two elements can combine to form two different compounds that have different proportions of those elements. 2.16(a) No, the percent by mass of each element in a compound is fixed. (b) Yes, the mass of each e ...
Document
... [1] In amorphous solid the particles (atoms, molecules or ions) are arranged in an irregular and non-repetitive three dimensional arrangements. [2] Rapidly solidified liquids are amorphous substances, e.g. Glass, rubber etc. [3] These solids are generally Isotropic. Note : Inotropic substances/Solid ...
... [1] In amorphous solid the particles (atoms, molecules or ions) are arranged in an irregular and non-repetitive three dimensional arrangements. [2] Rapidly solidified liquids are amorphous substances, e.g. Glass, rubber etc. [3] These solids are generally Isotropic. Note : Inotropic substances/Solid ...
Title
... 1(PF6)2 was obtained by reaction of 1Cl2 with NaPF6 in water. Its NMR spectrum is indistinguishable from the starting complex 1Cl2, confirming that the cationic complex 1 remains unaltered. The crystal structure obtained for 1(PF6)2 confirms the cisdisposition of the ligands (Fig. 7). The trans-isom ...
... 1(PF6)2 was obtained by reaction of 1Cl2 with NaPF6 in water. Its NMR spectrum is indistinguishable from the starting complex 1Cl2, confirming that the cationic complex 1 remains unaltered. The crystal structure obtained for 1(PF6)2 confirms the cisdisposition of the ligands (Fig. 7). The trans-isom ...
www.xtremepapers.net
... Specimen papers for Papers 31/32, 4 and 5 are available on the Teacher Support Site. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, Learning Outcomes have been used throughout. Each part of the syllabus is specified by a br ...
... Specimen papers for Papers 31/32, 4 and 5 are available on the Teacher Support Site. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, Learning Outcomes have been used throughout. Each part of the syllabus is specified by a br ...
Chapter 1 Chirality in clinical analysis 1.1. Introduction
... The term “Chirality” (from the Greek kheir for hand), means handedness, which is the existence of left/right opposites. The concept of “Chirality” has been known in chemistry since the 1870’s although a hundred years passed before chemists began using this term. In 1962, the first edition of Eliel’s ...
... The term “Chirality” (from the Greek kheir for hand), means handedness, which is the existence of left/right opposites. The concept of “Chirality” has been known in chemistry since the 1870’s although a hundred years passed before chemists began using this term. In 1962, the first edition of Eliel’s ...
Formation and interactions of cold and ultracold molecules
... molecule in a well-defined rovibrational level. It is recognized for a while now that the hyperfine interaction, i.e. the coupling between electronic and nuclear angular momenta, plays a crucial role in the photoassociation of cold atoms, especially when looking at molecular energy levels close to t ...
... molecule in a well-defined rovibrational level. It is recognized for a while now that the hyperfine interaction, i.e. the coupling between electronic and nuclear angular momenta, plays a crucial role in the photoassociation of cold atoms, especially when looking at molecular energy levels close to t ...
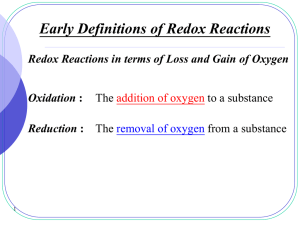
13.0 Redox Reactions PowerPoint
... • Now add the half-reactions and cancel the terms that appear on both sides of the equation to obtain the net-ionic equation 2 Ag+(aq) + 2 e- + Cu(s) 2 Ag(s) + Cu 2+ (aq) + 2 e2 Ag+(aq)+ Cu(s) 2 Ag(s) + Cu 2+ (aq) ▫ Silver ions are reduced to silver metal by reaction with copper metal. Simultane ...
... • Now add the half-reactions and cancel the terms that appear on both sides of the equation to obtain the net-ionic equation 2 Ag+(aq) + 2 e- + Cu(s) 2 Ag(s) + Cu 2+ (aq) + 2 e2 Ag+(aq)+ Cu(s) 2 Ag(s) + Cu 2+ (aq) ▫ Silver ions are reduced to silver metal by reaction with copper metal. Simultane ...
Some basic concepts of chemistry
... Further, a pure sample of copper carbonate was synthesized in laboratory and it was found that the percentage by weight of copper, carbon and oxygen were exactly identical to that of the naturally occurring sample of copper carbonate. c. French scientist Berthollet opposed Proust’s law of definite p ...
... Further, a pure sample of copper carbonate was synthesized in laboratory and it was found that the percentage by weight of copper, carbon and oxygen were exactly identical to that of the naturally occurring sample of copper carbonate. c. French scientist Berthollet opposed Proust’s law of definite p ...
CHAPTER-8 NCERT SOLUTIONS
... Adding the two half reactions, we have the net balanced redox reaction as: (b)Following the steps as in part (a), we have the oxidation half reaction as: And the reduction half reaction as: Multiplying the oxidation half reaction by 5 and the reduction half reaction by 2, and then by adding them, we ...
... Adding the two half reactions, we have the net balanced redox reaction as: (b)Following the steps as in part (a), we have the oxidation half reaction as: And the reduction half reaction as: Multiplying the oxidation half reaction by 5 and the reduction half reaction by 2, and then by adding them, we ...
57 estonian national chemistry olympiad
... One of the several allotropes of element A is a semiconductor. Generally, in compounds, element A is trivalent. Colourless gas B consists of element A and nonmetal X, used in cleaning means; molecular formula of B can be written as AX3. If gas B is heated in hydrogen atmosphere, elementary compound ...
... One of the several allotropes of element A is a semiconductor. Generally, in compounds, element A is trivalent. Colourless gas B consists of element A and nonmetal X, used in cleaning means; molecular formula of B can be written as AX3. If gas B is heated in hydrogen atmosphere, elementary compound ...
A Model For the Calculation of Solvent ... Reaction Rates for Process Design Purposes
... solvents be chosen with respect not only to their effectiveness in their respective process tasks but also for process-wide requirements such as their ease of recovery, low toxicity and environmental impact and possible applicability to other process tasks. Although there are models for the evaluati ...
... solvents be chosen with respect not only to their effectiveness in their respective process tasks but also for process-wide requirements such as their ease of recovery, low toxicity and environmental impact and possible applicability to other process tasks. Although there are models for the evaluati ...
Oxidation-Reduction Reactions - An Introduction to Chemistry
... Electrons are rarely found unattached to atoms. Thus, for one element or compound to lose electrons and be oxidized, another element or compound must be there to gain the electrons and be reduced. In other words, oxidation (loss of electrons) must be accompanied by reduction (gain of electrons). In ...
... Electrons are rarely found unattached to atoms. Thus, for one element or compound to lose electrons and be oxidized, another element or compound must be there to gain the electrons and be reduced. In other words, oxidation (loss of electrons) must be accompanied by reduction (gain of electrons). In ...
Exam Review Packet Table of Contents
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
PDF File
... observed distances between electron densities. The possible reasons for this difference have been discussed in the literature (10, 13, 16) and are not the subject of this report. Here we determine to what extent the observed ground state ribozyme environment around the catalytic metal ion, MA, is su ...
... observed distances between electron densities. The possible reasons for this difference have been discussed in the literature (10, 13, 16) and are not the subject of this report. Here we determine to what extent the observed ground state ribozyme environment around the catalytic metal ion, MA, is su ...
Spring 2008
... There are more molecules in the gas phase on the LH side, to increasing the P drives it to the right 27. For the reaction: aA(g) + bB(g) cC(g) + heat with a = 1, b=1 and c=3. An increase in total pressure (at const T) A. increases the equilibrium constant B. decreases the equilibrium constant C. doe ...
... There are more molecules in the gas phase on the LH side, to increasing the P drives it to the right 27. For the reaction: aA(g) + bB(g) cC(g) + heat with a = 1, b=1 and c=3. An increase in total pressure (at const T) A. increases the equilibrium constant B. decreases the equilibrium constant C. doe ...
Chapter 9 Reaction Energetics
... * Actually, the term mass-energy should be used instead of energy because mass and energy can be converted into one another by E = mc2. In nuclear reactions, where large amounts of energy are involved, there are substantial changes in mass. However, the mass changes in chemical processes are undetec ...
... * Actually, the term mass-energy should be used instead of energy because mass and energy can be converted into one another by E = mc2. In nuclear reactions, where large amounts of energy are involved, there are substantial changes in mass. However, the mass changes in chemical processes are undetec ...
File
... commonly thought of as a metal, does have some nonmetallic properties as its bonds to other nonmetals have significant covalent character. The other Group 3A elements have typical metal characteristics; its compounds formed with nonmetals are ionic. From this discussion, metallic character increases ...
... commonly thought of as a metal, does have some nonmetallic properties as its bonds to other nonmetals have significant covalent character. The other Group 3A elements have typical metal characteristics; its compounds formed with nonmetals are ionic. From this discussion, metallic character increases ...
DOE Chemistry 1
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
Redox reactions - SALEM-Immanuel Lutheran College
... bonds is equal to the charge it would have if it existed as an ion in that compound. E.g. H Cl , since Cl is more electronegative, the presumed electrical charges and thus O.N. of Cl and H are 1 and 1 respectively. ...
... bonds is equal to the charge it would have if it existed as an ion in that compound. E.g. H Cl , since Cl is more electronegative, the presumed electrical charges and thus O.N. of Cl and H are 1 and 1 respectively. ...
Disproportionation of Gold(II)
... showed this conclusion could be extended to neutral, threecoordinate AuI complexes of the type Au(phosphine)2X (X ) univalent anion, e.g., chloride, bromide or iodide). The results of these theoretical predictions have been utilized by the experimental group of Omary to design series of novel lumine ...
... showed this conclusion could be extended to neutral, threecoordinate AuI complexes of the type Au(phosphine)2X (X ) univalent anion, e.g., chloride, bromide or iodide). The results of these theoretical predictions have been utilized by the experimental group of Omary to design series of novel lumine ...