21 More About Amines • Heterocyclic Compounds
... in Chapter 30, when we take a look at how drugs are discovered and designed. Amines are also exceedingly important compounds to organic chemists, far too important to leave until the end of a course in organic chemistry. We have, therefore, already studied many aspects of amines and their chemistry. ...
... in Chapter 30, when we take a look at how drugs are discovered and designed. Amines are also exceedingly important compounds to organic chemists, far too important to leave until the end of a course in organic chemistry. We have, therefore, already studied many aspects of amines and their chemistry. ...
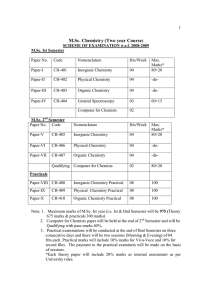
M.Sc. Chemistry (Two year Course)
... questions in all. Out of nine questions one question will be compulsory containing eight short answer type questions covering the entire syllabus. Further examiner will set two questions from each section and the candidates will be required to attempt one question from each section. All questions wi ...
... questions in all. Out of nine questions one question will be compulsory containing eight short answer type questions covering the entire syllabus. Further examiner will set two questions from each section and the candidates will be required to attempt one question from each section. All questions wi ...
Role of Chemical Reaction Engineering in Sustainable
... Alternative direct synthetic routes are therefore ...
... Alternative direct synthetic routes are therefore ...
ExamView - 1984 AP Chemistry Exam.tst
... Directions: Each set of lettered choices below refers to the numbered questions or statements immediately following it. Select the one lettered choice that best answers each question or best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, m ...
... Directions: Each set of lettered choices below refers to the numbered questions or statements immediately following it. Select the one lettered choice that best answers each question or best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, m ...
Solutions for Chapter 8 End-of-Chapter Problems
... The conclusion in Problem 8.10(c) is reinforced by these results: the more solute molecules in a given volume, the greater the number of arrangements. The same restriction applies here. To see the affect on W when n > N/2, calculate W for N = 20 and n = 9 and 11. Is the result at all surprising? Why ...
... The conclusion in Problem 8.10(c) is reinforced by these results: the more solute molecules in a given volume, the greater the number of arrangements. The same restriction applies here. To see the affect on W when n > N/2, calculate W for N = 20 and n = 9 and 11. Is the result at all surprising? Why ...
The polydentate ligands include polyaminopolycarbonic acids, such
... oxygen transport to the tissues of the body. Permanent exchange of substances to the environment enables to the body maintain a certain level of concentration of the compounds involved in the equilibrium of the complexation processes, providing metal-ligand homeostasis. In addition, complex compound ...
... oxygen transport to the tissues of the body. Permanent exchange of substances to the environment enables to the body maintain a certain level of concentration of the compounds involved in the equilibrium of the complexation processes, providing metal-ligand homeostasis. In addition, complex compound ...
aq - Haverford Alchemy
... (For example, it would be much more dangerous to spill a high concentration of hydrochloric acid on your hand than a low concentration) • Molarity is one way to measure the concentration of a ...
... (For example, it would be much more dangerous to spill a high concentration of hydrochloric acid on your hand than a low concentration) • Molarity is one way to measure the concentration of a ...
silbchp4
... A, In forming the ionic compound MgO, each Mg atom transfers two electrons to each O atom. (Note that atoms become smaller when they lose electrons and larger when they gain electrons.) The resulting Mg 2+ and O2- ions aggregate with many others to form an ionic solid. B, In the reactants H2 and Cl2 ...
... A, In forming the ionic compound MgO, each Mg atom transfers two electrons to each O atom. (Note that atoms become smaller when they lose electrons and larger when they gain electrons.) The resulting Mg 2+ and O2- ions aggregate with many others to form an ionic solid. B, In the reactants H2 and Cl2 ...
Oxidation numbers
... In the compound CO, the sum of the oxidation numbers must be 0 (rule 3). We know that oxygen has an oxidation number of −2 (this is not a peroxide) and since there is only one oxygen atom in the molecule, then the carbon atom must have an oxidation number of +2. So the oxidation number of carbon is ...
... In the compound CO, the sum of the oxidation numbers must be 0 (rule 3). We know that oxygen has an oxidation number of −2 (this is not a peroxide) and since there is only one oxygen atom in the molecule, then the carbon atom must have an oxidation number of +2. So the oxidation number of carbon is ...
chapter 3 Questions
... Avogadro's number of pages; calculate the thickness of the book in light-years. (Hint: A light year is the distance traveled by light in one year, or 365 days, at a speed of 3.00 108 m/s) A) 2.2 1021 light-yr B) 6.0 1023 light-yr C) 5.8 103 light-yr D) 3.4 1016 light-yr ...
... Avogadro's number of pages; calculate the thickness of the book in light-years. (Hint: A light year is the distance traveled by light in one year, or 365 days, at a speed of 3.00 108 m/s) A) 2.2 1021 light-yr B) 6.0 1023 light-yr C) 5.8 103 light-yr D) 3.4 1016 light-yr ...
Synthesis and Structural Studies of Calcium and Magnesium
... growth including hydrothermal methods and gel crystallization were employed. We have used phosphinate and phosphonate ligands with different number of phosphorus oxygen atoms as well as diphosphonates with different linker lengths to determine their effects on the overall structural features. An int ...
... growth including hydrothermal methods and gel crystallization were employed. We have used phosphinate and phosphonate ligands with different number of phosphorus oxygen atoms as well as diphosphonates with different linker lengths to determine their effects on the overall structural features. An int ...
Chapter 4
... In order for there to be an electric current through each solution, cations and anions must be present and must be free to migrate between the two graphite electrodes. In the microscopic view of each solution, solvent molecules are not shown because we want to focus on the solutes; the blue backgrou ...
... In order for there to be an electric current through each solution, cations and anions must be present and must be free to migrate between the two graphite electrodes. In the microscopic view of each solution, solvent molecules are not shown because we want to focus on the solutes; the blue backgrou ...
coordination compounds
... oxygen transport to the tissues of the body. Permanent exchange of substances to the environment enables to the body maintain a certain level of concentration of the compounds involved in the equilibrium of the complexation processes, providing metal-ligand homeostasis. In addition, complex compound ...
... oxygen transport to the tissues of the body. Permanent exchange of substances to the environment enables to the body maintain a certain level of concentration of the compounds involved in the equilibrium of the complexation processes, providing metal-ligand homeostasis. In addition, complex compound ...
Atomic orbitals, symmetry, and coordination polyhedra
... angular coordinates u and f, it is independent of direction (i.e., isotropic) and therefore remains unaltered by any symmetry operations. For this reason all of the symmetry properties of a spherical harmonic C, and thus of the corresponding wave function or atomic orbital, are contained in its angu ...
... angular coordinates u and f, it is independent of direction (i.e., isotropic) and therefore remains unaltered by any symmetry operations. For this reason all of the symmetry properties of a spherical harmonic C, and thus of the corresponding wave function or atomic orbital, are contained in its angu ...
Water deuteration and ortho-to-para nuclear spin ratio of H2 in
... time-dependent cooling/heating rates and simplified chemistry (B04). The cosmic-ray ionization rate of H2 (ξH2 ) is set to be 1.3 × 10−17 s−1 . Interstellar radiation is assumed to be incident from one side only, adopting the Draine field (Draine 1978). As time proceeds, the column density of post-s ...
... time-dependent cooling/heating rates and simplified chemistry (B04). The cosmic-ray ionization rate of H2 (ξH2 ) is set to be 1.3 × 10−17 s−1 . Interstellar radiation is assumed to be incident from one side only, adopting the Draine field (Draine 1978). As time proceeds, the column density of post-s ...
the chemical and physical properties of condensed
... functional groups are all similar in behaviour and respond to change in their environment in a similar manner. All known metaphosphate ring systems can be easily crystallized as stable alkali metal salts. The polyphosphates, convefsely, are highly unsymmetrical in both structure and property. There ...
... functional groups are all similar in behaviour and respond to change in their environment in a similar manner. All known metaphosphate ring systems can be easily crystallized as stable alkali metal salts. The polyphosphates, convefsely, are highly unsymmetrical in both structure and property. There ...
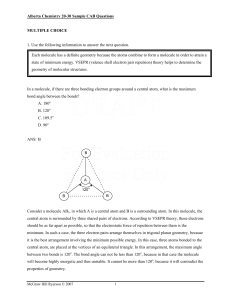
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
Document
... Predicting a precipitation reaction and writing the chemical equation 1 Write the reactants for the reaction 2 Show the reactants in their ionic form, i.e., if they are soluble, write their dissociated form 3 Determine which cation-anion combination yields an insoluble compound ...
... Predicting a precipitation reaction and writing the chemical equation 1 Write the reactants for the reaction 2 Show the reactants in their ionic form, i.e., if they are soluble, write their dissociated form 3 Determine which cation-anion combination yields an insoluble compound ...
Packet 1 - Kentucky Community and Technical College System
... Step 3 Use the solubility rules to decide whether a solid forms and, if so, to predict the identity of the solid. CHE 170 Packet 4 - 50 ...
... Step 3 Use the solubility rules to decide whether a solid forms and, if so, to predict the identity of the solid. CHE 170 Packet 4 - 50 ...
Calculations on the equations reaction
... valences this element can have in compounds? Write the formula of highest oxide of this element. 2. An element has serial number 19 define: а) charge of nucleus atom b) number of electrons c) number of neutrons and protons. Write electronic formula of element. What valences this element can have in ...
... valences this element can have in compounds? Write the formula of highest oxide of this element. 2. An element has serial number 19 define: а) charge of nucleus atom b) number of electrons c) number of neutrons and protons. Write electronic formula of element. What valences this element can have in ...
Stoichiometry
... formed. The reaction will stop when all of the limiting reactant is consumed. Example: I want to assemble a gadget that requires one nut, one bolt and two washers for every hole. I have in my garage a bucket filled with 12 washers, 4 bolts and five nuts. What is the LIMITING SMALL METAL ...
... formed. The reaction will stop when all of the limiting reactant is consumed. Example: I want to assemble a gadget that requires one nut, one bolt and two washers for every hole. I have in my garage a bucket filled with 12 washers, 4 bolts and five nuts. What is the LIMITING SMALL METAL ...