Regents Chemistry Review - New York Science Teacher
... The table gives information about the nucleus of each of four atoms. • How many different elements are represented by the nuclei in the table? ...
... The table gives information about the nucleus of each of four atoms. • How many different elements are represented by the nuclei in the table? ...
Reactants Products
... concentration of NO2 doubles, the concentration of CO stays constant, and the rate quadruples, suggesting that the reaction is second order in NO2. Between the second and third experiments, the concentration of NO2 stays constant, the concentration of CO doubles, and the rate remains constant (the s ...
... concentration of NO2 doubles, the concentration of CO stays constant, and the rate quadruples, suggesting that the reaction is second order in NO2. Between the second and third experiments, the concentration of NO2 stays constant, the concentration of CO doubles, and the rate remains constant (the s ...
Unit 1 Mole and enthalpy changes
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
Mole-Volume Conversion Assignment
... In most chemical reactions one reactant is in excess and one is limiting. Deciding which reactants are the limiting reagents and the reactants in excess when given masses of both reactants: 1. Write the balanced chemical equation for the chemical reaction 2. Determine how many moles of each reactant ...
... In most chemical reactions one reactant is in excess and one is limiting. Deciding which reactants are the limiting reagents and the reactants in excess when given masses of both reactants: 1. Write the balanced chemical equation for the chemical reaction 2. Determine how many moles of each reactant ...
1.1 Applications of Cold Molecules
... The dipole moment is a measure for the charge distribution of the particle. Since the electron is assumed to be a point charge, however, the EDM is considered to be a measure of the average displacement of the electron charge from the electron’s center of mass. An EDM would imply an asymmetry with r ...
... The dipole moment is a measure for the charge distribution of the particle. Since the electron is assumed to be a point charge, however, the EDM is considered to be a measure of the average displacement of the electron charge from the electron’s center of mass. An EDM would imply an asymmetry with r ...
Chemistry
... materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such as cookware), liquid crystals (like those in electronic displays), adhesives (used in your Post-It notes), and ...
... materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such as cookware), liquid crystals (like those in electronic displays), adhesives (used in your Post-It notes), and ...
Mole and Energy - Deans Community High School
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
... Note: It is essential to show the division by the volume, V dm3, even though they cancel, to show that you have understood that the terms in the expression are concentrations. Also, it is essential to state that there are no units, since the question demands that you state the units, if any. Lastly, ...
... Note: It is essential to show the division by the volume, V dm3, even though they cancel, to show that you have understood that the terms in the expression are concentrations. Also, it is essential to state that there are no units, since the question demands that you state the units, if any. Lastly, ...
reaction rate - davis.k12.ut.us
... Expressing Reaction Rates (cont.) • Reaction rates are determined experimentally (and always expressed as a positive value). • Average rate of reaction: the change in concentration of a reactant or product that occurs during a specific time interval. ...
... Expressing Reaction Rates (cont.) • Reaction rates are determined experimentally (and always expressed as a positive value). • Average rate of reaction: the change in concentration of a reactant or product that occurs during a specific time interval. ...
Organic Chemistry with a Biological Emphasis Volume I
... Composed of the four elements carbon, hydrogen, oxygen and nitrogen, capsaicin is produced by the pepper plant for the purpose of warding off hungry mammals. The molecule binds to and activates a mammalian receptor protein called TrpV1, which in normal circumstances has the job of detecting high tem ...
... Composed of the four elements carbon, hydrogen, oxygen and nitrogen, capsaicin is produced by the pepper plant for the purpose of warding off hungry mammals. The molecule binds to and activates a mammalian receptor protein called TrpV1, which in normal circumstances has the job of detecting high tem ...
Title Photo-Induced Electron Transfer Between a Reactant Molecule
... Photocatalysis of semiconductor-type metal oxides is generally explained in terms of band theory (the classical electron transfer mechanism) accompanied by the interaction of reactants with the photo-generated electrons and holes, and is potentially available to make the catalytic reaction proceedin ...
... Photocatalysis of semiconductor-type metal oxides is generally explained in terms of band theory (the classical electron transfer mechanism) accompanied by the interaction of reactants with the photo-generated electrons and holes, and is potentially available to make the catalytic reaction proceedin ...
File
... Solid xenon hexafluoride is prepared by allowing xenon gas and fluorine gas to react. Xe (g) + 3F2 (g) XeF6 (s) ◦ How many grams of fluorine are required to produce 10.0 g of XeF6? ◦ How many grams of xenon are required to produce 10.0 g of XeF6? ...
... Solid xenon hexafluoride is prepared by allowing xenon gas and fluorine gas to react. Xe (g) + 3F2 (g) XeF6 (s) ◦ How many grams of fluorine are required to produce 10.0 g of XeF6? ◦ How many grams of xenon are required to produce 10.0 g of XeF6? ...
Final Exam - KFUPM Faculty List
... on the left the charge is 7+, on the right 0. To balance it, we add 7 e- to the left. Reduction: 7 e- + BrO4-(aq) + 8 H+(aq) 1/2 Br2(l) + 4 H2O(l) Perbromate is reduced and thus is the oxidizing agent. Bromide is simply oxidized: Br-(aq) 1/2 Br2 Adding an electron on the right (oxidation) balanc ...
... on the left the charge is 7+, on the right 0. To balance it, we add 7 e- to the left. Reduction: 7 e- + BrO4-(aq) + 8 H+(aq) 1/2 Br2(l) + 4 H2O(l) Perbromate is reduced and thus is the oxidizing agent. Bromide is simply oxidized: Br-(aq) 1/2 Br2 Adding an electron on the right (oxidation) balanc ...
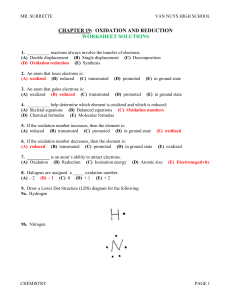
Solutions_C19
... For CO: Oxygen has a – 2 oxidation number. Therefore, carbon must have a + 2 oxidation number. For SO3: Oxygen has a – 2 oxidation number. The three oxygen atoms have a combined – 6 oxidation number. Sulfur must have a + 6 oxidation number to balance out the molecule. Final oxidation numbers C(+4)O2 ...
... For CO: Oxygen has a – 2 oxidation number. Therefore, carbon must have a + 2 oxidation number. For SO3: Oxygen has a – 2 oxidation number. The three oxygen atoms have a combined – 6 oxidation number. Sulfur must have a + 6 oxidation number to balance out the molecule. Final oxidation numbers C(+4)O2 ...
Metal Complexes Containing Natural and Artificial Radioactive
... the Ln3+ ions (ca. 1,000 cm−1). In contrast to lanthanides, J is comparable with the ligand-field splitting and is no longer a good quantum number. The proximity of the energy of the 5f and 6d orbitals and the population of thermally accessible excited levels lead to the expression for effective mag ...
... the Ln3+ ions (ca. 1,000 cm−1). In contrast to lanthanides, J is comparable with the ligand-field splitting and is no longer a good quantum number. The proximity of the energy of the 5f and 6d orbitals and the population of thermally accessible excited levels lead to the expression for effective mag ...
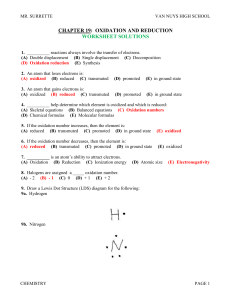
Solutions_C19
... For CO: Oxygen has a – 2 oxidation number. Therefore, carbon must have a + 2 oxidation number. For SO3: Oxygen has a – 2 oxidation number. The three oxygen atoms have a combined – 6 oxidation number. Sulfur must have a + 6 oxidation number to balance out the molecule. Final oxidation numbers C(+4)O2 ...
... For CO: Oxygen has a – 2 oxidation number. Therefore, carbon must have a + 2 oxidation number. For SO3: Oxygen has a – 2 oxidation number. The three oxygen atoms have a combined – 6 oxidation number. Sulfur must have a + 6 oxidation number to balance out the molecule. Final oxidation numbers C(+4)O2 ...
Chapter 14
... mol/L, it takes __________ s for the concentration to decrease to 0.11 mol/L. A) 0.017 B) 0.68 C) 9.1 D) 40. E) 5.2 Chapter 15 Chemical Equilibrium 1) At equilibrium, __________. A) all chemical reactions have ceased B) the rates of the forward and reverse reactions are equal C) the rate constants o ...
... mol/L, it takes __________ s for the concentration to decrease to 0.11 mol/L. A) 0.017 B) 0.68 C) 9.1 D) 40. E) 5.2 Chapter 15 Chemical Equilibrium 1) At equilibrium, __________. A) all chemical reactions have ceased B) the rates of the forward and reverse reactions are equal C) the rate constants o ...
1.8 M - Thierry Karsenti
... pure substances 3. The chemical combination of three different kinds of atoms results in the formation of a) a single large atom c) a molecule b) an element d) a compound 4. Which of the following is correct about heat and temperature? a) Samples of the same substance at the same temperature will h ...
... pure substances 3. The chemical combination of three different kinds of atoms results in the formation of a) a single large atom c) a molecule b) an element d) a compound 4. Which of the following is correct about heat and temperature? a) Samples of the same substance at the same temperature will h ...
Part A Completion
... © Pearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved. ...
... © Pearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved. ...