Preparation of Reducing Sugar Hydrolyzed from High
... In this investigation, XRD patterns were investigated at distinct three angles, 2θ = 16.00o, 22.00o, and 35.00o, which correlated to reflector planes of (101), (002) and (040) in the cellulosic crystal as shown in Figure 6 [20-22,25-26]. After pretreatment with one percent alkaline (1% NaOH) and rec ...
... In this investigation, XRD patterns were investigated at distinct three angles, 2θ = 16.00o, 22.00o, and 35.00o, which correlated to reflector planes of (101), (002) and (040) in the cellulosic crystal as shown in Figure 6 [20-22,25-26]. After pretreatment with one percent alkaline (1% NaOH) and rec ...
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... ¾ Atoms of one element are different from atoms of all other elements. ¾ Different properties explained by assuming atoms are not the same type The second hypothesis: ¾ To form a certain compound, need atoms of right elements, and proportion. ¾ Extension of a law published in 1799 by Joseph Proust ¾ ...
... ¾ Atoms of one element are different from atoms of all other elements. ¾ Different properties explained by assuming atoms are not the same type The second hypothesis: ¾ To form a certain compound, need atoms of right elements, and proportion. ¾ Extension of a law published in 1799 by Joseph Proust ¾ ...
DELTAHPP
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
No Slide Title
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
enthalpy changes
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
Chem Course Desc2. New
... your life more comfortable. Chemistry is needed to understand many processes in a variety of industries: pharmaceuticals, cosmetics, petroleum, plastics, food technology, etc. Chemistry is also the basis of all life on Earth, from bacteria to human beings and everything in between. In our most basic ...
... your life more comfortable. Chemistry is needed to understand many processes in a variety of industries: pharmaceuticals, cosmetics, petroleum, plastics, food technology, etc. Chemistry is also the basis of all life on Earth, from bacteria to human beings and everything in between. In our most basic ...
The Process of Chemical Reactions
... Gasoline and oxygen coexist quietly until a spark from a spark plug propels them into violent reaction. Why? Why are ozone molecules in the stratosphere destroyed more rapidly in the presence of chlorine atoms released from CFC molecules? In order to understand these situations, we need to take a lo ...
... Gasoline and oxygen coexist quietly until a spark from a spark plug propels them into violent reaction. Why? Why are ozone molecules in the stratosphere destroyed more rapidly in the presence of chlorine atoms released from CFC molecules? In order to understand these situations, we need to take a lo ...
U3 Student Workbook - The Connected Chemistry Curriculum
... This lesson contains four activities that introduce students to five different kinds of chemical reactions (combination, decomposition, single displacement, double displacement, and combustion reactions). In the Connecting Activity, students apply the Law of Conservation of Mass to chemical equation ...
... This lesson contains four activities that introduce students to five different kinds of chemical reactions (combination, decomposition, single displacement, double displacement, and combustion reactions). In the Connecting Activity, students apply the Law of Conservation of Mass to chemical equation ...
IIT-JEE (Advanced) - Brilliant Public School Sitamarhi
... Q.15 A sample containing only CaCO3 and MgCO3 is ignited to CaO and MgO. The mixture of oxides produced weight exactly half as much as the original sample. Calculate the percentages of CaCO3 and MgCO3 in the sample. Q.16 Determine the percentage composition of a mixture of anhydrous sodium carbonate ...
... Q.15 A sample containing only CaCO3 and MgCO3 is ignited to CaO and MgO. The mixture of oxides produced weight exactly half as much as the original sample. Calculate the percentages of CaCO3 and MgCO3 in the sample. Q.16 Determine the percentage composition of a mixture of anhydrous sodium carbonate ...
The Process of Chemical Reactions
... Gasoline and oxygen coexist quietly until a spark from a spark plug propels them into violent reaction. Why? Why are ozone molecules in the stratosphere destroyed more rapidly in the presence of chlorine atoms released from CFC molecules? In order to understand these situations, we need to take a lo ...
... Gasoline and oxygen coexist quietly until a spark from a spark plug propels them into violent reaction. Why? Why are ozone molecules in the stratosphere destroyed more rapidly in the presence of chlorine atoms released from CFC molecules? In order to understand these situations, we need to take a lo ...
2016-2018 Syllabus - Cambridge International Examinations
... Part A and Part B of the syllabus. Details of the new scheme of assessment can be found on page 5. If there are any further changes to this syllabus, Cambridge will write to Centres to inform them. This syllabus is also on the Cambridge website www.cie.org.uk/cambridgepreu. The version of the syllab ...
... Part A and Part B of the syllabus. Details of the new scheme of assessment can be found on page 5. If there are any further changes to this syllabus, Cambridge will write to Centres to inform them. This syllabus is also on the Cambridge website www.cie.org.uk/cambridgepreu. The version of the syllab ...
deltahpps
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
... making bonds is exothermic as it is the opposite of breaking a bond for diatomic gases, bond enthalpy = 2 x enthalpy of atomisation smaller bond enthalpy = weaker bond = easier to break ...
- Kendriya Vidyalaya Jamuna Colliery
... Mass of Niobium = 92.9u, NA = 6.022 x 1023 atoms mol-1 ]. 4. If radius of octahedral void is r and radius of atom in close packing is R, derive the relationship between r and R. 5. Non stoichiometric cuprous oxide can be prepared in the laboratory. In this oxide, copper to oxygen ratio is slightly l ...
... Mass of Niobium = 92.9u, NA = 6.022 x 1023 atoms mol-1 ]. 4. If radius of octahedral void is r and radius of atom in close packing is R, derive the relationship between r and R. 5. Non stoichiometric cuprous oxide can be prepared in the laboratory. In this oxide, copper to oxygen ratio is slightly l ...
File - Junior College Chemistry tuition
... The capacity of a cell is given in amp hours (Ah), which is the amount of constant current that can be drawn from the cell in one hour before it becomes discharged. Which expression will calculate the minimum mass of lithium required to make a cell with a capacity of 1.8 Ah? A ...
... The capacity of a cell is given in amp hours (Ah), which is the amount of constant current that can be drawn from the cell in one hour before it becomes discharged. Which expression will calculate the minimum mass of lithium required to make a cell with a capacity of 1.8 Ah? A ...
CHEM 121 Chp 5 Spaulding
... weights of all the atoms in a compound, reported in atomic mass units The molar mass is the mass of one mole of any substance, reported in grams ◦ The value of the molar mass of a compound in grams equals the value of its formula weight in ...
... weights of all the atoms in a compound, reported in atomic mass units The molar mass is the mass of one mole of any substance, reported in grams ◦ The value of the molar mass of a compound in grams equals the value of its formula weight in ...
organonitrogen compounds i. amines
... 23-5A lnfrared and Ultraviolet Spectra A characteristic feature of the infrared spectra of primary and secondary amines is the moderately weak absorption at 3500 cm-l to 3300 cm-l, which corresponds to N-H stretching vibrations. Primary amines have two such bands in this region, whereas secondary am ...
... 23-5A lnfrared and Ultraviolet Spectra A characteristic feature of the infrared spectra of primary and secondary amines is the moderately weak absorption at 3500 cm-l to 3300 cm-l, which corresponds to N-H stretching vibrations. Primary amines have two such bands in this region, whereas secondary am ...
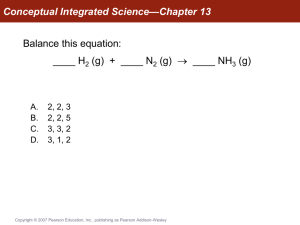
Conceptual Integrated Science—Chapter 13
... an orbiting space station because there is no up or down. As a result, what happens to the burning candle and why? A. The warm air surrounding the candle speeds up the rate of reaction so that the candle burns brighter. B. Soot from the candle forms a ball around the candle. The temperature inside t ...
... an orbiting space station because there is no up or down. As a result, what happens to the burning candle and why? A. The warm air surrounding the candle speeds up the rate of reaction so that the candle burns brighter. B. Soot from the candle forms a ball around the candle. The temperature inside t ...
chapter 16
... Step 2 Formation of activated complex: As the oxygen atoms of the O−O bond in the O3 molecule separate, the attraction between them decreases, and as the attraction decreases, less energy is needed for moving the atoms even farther apart. Meanwhile, the oxygen atoms that are forming the new bond mov ...
... Step 2 Formation of activated complex: As the oxygen atoms of the O−O bond in the O3 molecule separate, the attraction between them decreases, and as the attraction decreases, less energy is needed for moving the atoms even farther apart. Meanwhile, the oxygen atoms that are forming the new bond mov ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... (For example, it would be much more dangerous to spill a high concentration of hydrochloric acid on your hand than a low concentration) • Molarity is one way to measure the concentration of a ...
... (For example, it would be much more dangerous to spill a high concentration of hydrochloric acid on your hand than a low concentration) • Molarity is one way to measure the concentration of a ...
Descriptive Inorganic Chemistry
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
Chemistry Higher Level Chapter 5 - Pearson Schools and FE Colleges
... information that one mole of methane gas reacts with two moles of oxygen gas to give one mole of gaseous carbon dioxide and two moles of liquid water and releases 890 kJ of heat energy. A few reactions are endothermic as they result in an energy transfer from the surroundings to the system. In this ...
... information that one mole of methane gas reacts with two moles of oxygen gas to give one mole of gaseous carbon dioxide and two moles of liquid water and releases 890 kJ of heat energy. A few reactions are endothermic as they result in an energy transfer from the surroundings to the system. In this ...
Chapter 5: Calculations and the Chemical Equation
... determining the composition. For example, dihydrogen monoxide (water, H2O) is a compound composed of two hydrogen atoms for every oxygen atom. The Chemical Formula A chemical formula (also called molecular formula) is a concise way of expressing information about the atoms that constitute a particul ...
... determining the composition. For example, dihydrogen monoxide (water, H2O) is a compound composed of two hydrogen atoms for every oxygen atom. The Chemical Formula A chemical formula (also called molecular formula) is a concise way of expressing information about the atoms that constitute a particul ...
Conformational studies of aliphatic secondary ozonides
... Conformational diversity of secondary ozonides of 1-alkenes is influenced by three different factors. Foremost, the five membered ring can adopt various puckering forms. The conformations of such a ring can be classified into an envelope (E) form in which one atom lies out of the plane of the other atom ...
... Conformational diversity of secondary ozonides of 1-alkenes is influenced by three different factors. Foremost, the five membered ring can adopt various puckering forms. The conformations of such a ring can be classified into an envelope (E) form in which one atom lies out of the plane of the other atom ...