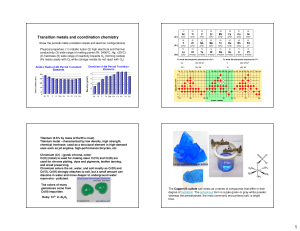
Final Exam Study Guide Word document
... How many molecules are in 237 grams (about a cup) of water? 18. What are the coefficients when the following chemical equation is balanced? AlCl 3 + H2SO4 ----> ...
... How many molecules are in 237 grams (about a cup) of water? 18. What are the coefficients when the following chemical equation is balanced? AlCl 3 + H2SO4 ----> ...
Regular Chemistry - 1st Semester Final Practice Exam
... 26) Which state of matter has atomic spacing that is far apart and definite shape? A) solid B) plasma C) gas D) liquid E) none of the above 27) Molten (liquid) iron metal has a(n) ________ volume and a(n) ________ shape. A) indefinite; definite B) definite; definite C) definite; indefinite D) indef ...
... 26) Which state of matter has atomic spacing that is far apart and definite shape? A) solid B) plasma C) gas D) liquid E) none of the above 27) Molten (liquid) iron metal has a(n) ________ volume and a(n) ________ shape. A) indefinite; definite B) definite; definite C) definite; indefinite D) indef ...
Final Exam SG Part 1 (Unit 5).
... c. How many moles are produced from the moles of the reactants? d. If you double the amount of white molecules (so now you have 8 pairs) but keep the same amount of black molecules, how many molecules can you produce? 4. Percent Yield a. ___Sb4O6 + ____C → ____Sb + ____CO Determine the percent yield ...
... c. How many moles are produced from the moles of the reactants? d. If you double the amount of white molecules (so now you have 8 pairs) but keep the same amount of black molecules, how many molecules can you produce? 4. Percent Yield a. ___Sb4O6 + ____C → ____Sb + ____CO Determine the percent yield ...
PRACTICE PROBLEMS EXAM 1,2 and 3 1311
... 16) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) H, Li B) Cs, Ba C) Ca, Sr D) Ga, Ge E) C, O 17) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) O, S ...
... 16) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) H, Li B) Cs, Ba C) Ca, Sr D) Ga, Ge E) C, O 17) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) O, S ...
Chapter 9 - HCC Learning Web
... 60. Nitrous oxide, N2O, is sometimes called "laughing gas". What is the formal charge on the central nitrogen atom in the best Lewis structure for nitrous oxide? (The atom connectivity is N-N-O.) A. -2 B. -1 C. 0 D. +1 E. +2 62. In the Lewis structure of the iodate ion, IO3-, that satisfies the octe ...
... 60. Nitrous oxide, N2O, is sometimes called "laughing gas". What is the formal charge on the central nitrogen atom in the best Lewis structure for nitrous oxide? (The atom connectivity is N-N-O.) A. -2 B. -1 C. 0 D. +1 E. +2 62. In the Lewis structure of the iodate ion, IO3-, that satisfies the octe ...
CHAPTER 2. THE ELEMENTS: BASIC BUILDING BLOCKS OF …
... • The one electron in hydrogen, H, goes into the first electron shell, the one with the lowest possible energy The lowest electron shell can contain a maximum of only 2 electrons • So helium has a filled electron shell making it a noble gas Helium is a nontoxic, odorless, tasteless, colorless gas wi ...
... • The one electron in hydrogen, H, goes into the first electron shell, the one with the lowest possible energy The lowest electron shell can contain a maximum of only 2 electrons • So helium has a filled electron shell making it a noble gas Helium is a nontoxic, odorless, tasteless, colorless gas wi ...
Chemistry
... 9 – 13 Understand how pH is related to the molarity of the hydronium ion in an aqueous solution using logarithms. 9 – 14 Know how to do an acid/base titration using a standard solution to determine the concentration of an unknown solution. 10. Patterns of behavior ...
... 9 – 13 Understand how pH is related to the molarity of the hydronium ion in an aqueous solution using logarithms. 9 – 14 Know how to do an acid/base titration using a standard solution to determine the concentration of an unknown solution. 10. Patterns of behavior ...
vsepr
... regions occur on the central atom in a structure. Once you know this number, everything else becomes dependent upon it. In VSEPR Theory, we use this number to determine what the electron geometry will be – i.e., the geometry formed when the electron regions are as far apart from each other as possib ...
... regions occur on the central atom in a structure. Once you know this number, everything else becomes dependent upon it. In VSEPR Theory, we use this number to determine what the electron geometry will be – i.e., the geometry formed when the electron regions are as far apart from each other as possib ...
Practice problems for chapter 1, 2 and 3 1) A small amount of salt
... 16) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) H, Li B) Cs, Ba C) Ca, Sr D) Ga, Ge E) C, O 17) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) O, S ...
... 16) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) H, Li B) Cs, Ba C) Ca, Sr D) Ga, Ge E) C, O 17) Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties? A) O, S ...
Final Review 2006
... c. neutral group of atoms held together by covalent bonds. d. neutral group of atoms held together by ionic bonds. ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avo ...
... c. neutral group of atoms held together by covalent bonds. d. neutral group of atoms held together by ionic bonds. ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avo ...
Ionic Bond - hrsbstaff.ednet.ns.ca
... • The outer most electron shell in any atom is called the valence shell • The electrons in the valence shell are called Valence Electrons • By looking at the number of valence electrons an element has we can predict its reactivity. • THE OCTET RULE: Atoms will try to lose, gain or share electrons to ...
... • The outer most electron shell in any atom is called the valence shell • The electrons in the valence shell are called Valence Electrons • By looking at the number of valence electrons an element has we can predict its reactivity. • THE OCTET RULE: Atoms will try to lose, gain or share electrons to ...
AP Chemistry Unit 1 Essential Questions Screencast 1
... 3. What does a subscript in a chemical formula indicate? 4. What is gained or lost when an ion is formed? 5. Where are the elements that form cations located on the periodic table? Anions? 6. How is an ionic bond formed? Screencast 1-10 Ionic Compounds and Ionic Nomenclature 1. How is an ionic compo ...
... 3. What does a subscript in a chemical formula indicate? 4. What is gained or lost when an ion is formed? 5. Where are the elements that form cations located on the periodic table? Anions? 6. How is an ionic bond formed? Screencast 1-10 Ionic Compounds and Ionic Nomenclature 1. How is an ionic compo ...
File
... Chemical reactions involve either the transfer or the sharing of electrons between atoms. Therefore, the chemical reactivity/ properties of an element is primarily dependent upon the number of electrons in an atom of that element. Protons also play a significant role because the tendency for an atom ...
... Chemical reactions involve either the transfer or the sharing of electrons between atoms. Therefore, the chemical reactivity/ properties of an element is primarily dependent upon the number of electrons in an atom of that element. Protons also play a significant role because the tendency for an atom ...
Chem 173: Final Exam Review Short Answer and Problems 1
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C 2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate an ...
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C 2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate an ...
Document
... atoms of different size, or when the bonding to one atom is different than the bonding to another, this will affect the molecular geometry around the central atom. • Lone pairs also affect the molecular geometry. – They occupy space on the central atom, but are not “seen” as points on the molecular ...
... atoms of different size, or when the bonding to one atom is different than the bonding to another, this will affect the molecular geometry around the central atom. • Lone pairs also affect the molecular geometry. – They occupy space on the central atom, but are not “seen” as points on the molecular ...
Chapter 8 Concepts of Chemical Bonding
... Which of these molecules has the same number of shared electron pairs as unshared electron pairs? (a) HCl, (b) H2S, (c) PF3, (d) CCl2F2 (e) Br2. Practice Exercise 2 Compare the Lewis symbol for neon with the Lewis structure for methane, CH4. How many valence electrons are in each structure? How many ...
... Which of these molecules has the same number of shared electron pairs as unshared electron pairs? (a) HCl, (b) H2S, (c) PF3, (d) CCl2F2 (e) Br2. Practice Exercise 2 Compare the Lewis symbol for neon with the Lewis structure for methane, CH4. How many valence electrons are in each structure? How many ...
chem eng-problems
... 2) Complete the following equations and determine whether or not a precipitation reaction (exchange reaction) will occur. If a reaction will occur, balance the equation and 1) Determine the products of exchange reaction 2) Determine if there is any insoluble product 3) Balance the chemical equation ...
... 2) Complete the following equations and determine whether or not a precipitation reaction (exchange reaction) will occur. If a reaction will occur, balance the equation and 1) Determine the products of exchange reaction 2) Determine if there is any insoluble product 3) Balance the chemical equation ...
Name ionic compounds containing main group or
... Construct electron dot formulas to illustrate ionic and covalent bonds Predict molecular structure for molecules with linear, trigonal planar, or tetrahedral electron pair geometries using Valence Shell Electron Pair Repulsion (VSEPR) theory. 7. Be able to write Lewis dot structures for molecules sh ...
... Construct electron dot formulas to illustrate ionic and covalent bonds Predict molecular structure for molecules with linear, trigonal planar, or tetrahedral electron pair geometries using Valence Shell Electron Pair Repulsion (VSEPR) theory. 7. Be able to write Lewis dot structures for molecules sh ...
IPC – First Semester Exam Review Be able to classify an example
... To form the compound salt (NaCl), atoms of the elements, sodium (Na) and chlorine (Cl), form an ionic bond together. 3. What is the ratio of the sodium and chlorine atoms in salt? 1:1 ...
... To form the compound salt (NaCl), atoms of the elements, sodium (Na) and chlorine (Cl), form an ionic bond together. 3. What is the ratio of the sodium and chlorine atoms in salt? 1:1 ...
Utah - Wavefunction, Inc.
... Solutions make up many of the ordinary substances encountered in everyday life. The relative amounts of solutes and solvents determine the concentration and the physical properties of a solution. Two important categories of solutions are acids and bases. STANDARD VI: S ...
... Solutions make up many of the ordinary substances encountered in everyday life. The relative amounts of solutes and solvents determine the concentration and the physical properties of a solution. Two important categories of solutions are acids and bases. STANDARD VI: S ...