Chem12 SM Unit 5 Review final ok
... 42. (a) In P2O5, the oxidation number of O is –2 and the oxidation number of P is +5. (b) In NO2, the oxidation number of O is –2 and the oxidation number of N is +4. (c) In Na2SO4, the oxidation number of Na is +1, the oxidation number of O is –2, and the oxidation number of S is +6. (d) In Cu(NO3) ...
... 42. (a) In P2O5, the oxidation number of O is –2 and the oxidation number of P is +5. (b) In NO2, the oxidation number of O is –2 and the oxidation number of N is +4. (c) In Na2SO4, the oxidation number of Na is +1, the oxidation number of O is –2, and the oxidation number of S is +6. (d) In Cu(NO3) ...
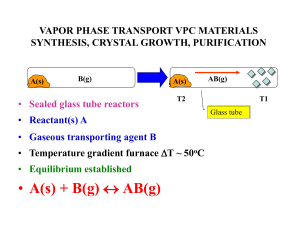
13 CHEMICAL EQUILIBRIUM W MODULE - 5
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
Hydrolases as Catalysts for Green Chemistry and
... The use of esterase has been investigated to provide an alternative clean route for the synthesis of a chiral pharmaceutical compound, S-clopidogrel, by selective hydrolysis of the racemic precursor. Current production of the pure S- clopidogrel isomer involves the use of a resolving agent, L-campho ...
... The use of esterase has been investigated to provide an alternative clean route for the synthesis of a chiral pharmaceutical compound, S-clopidogrel, by selective hydrolysis of the racemic precursor. Current production of the pure S- clopidogrel isomer involves the use of a resolving agent, L-campho ...
Recycling and Chemical Mathematics
... release oxygen gas in the process. The animal life of Biosphere 2, through the process of breathing (respiration), takes in atmospheric oxygen and releases carbon dioxide. If everything could be arranged to come out even, a stable atmosphere with desirable levels of oxygen and carbon dioxide would b ...
... release oxygen gas in the process. The animal life of Biosphere 2, through the process of breathing (respiration), takes in atmospheric oxygen and releases carbon dioxide. If everything could be arranged to come out even, a stable atmosphere with desirable levels of oxygen and carbon dioxide would b ...
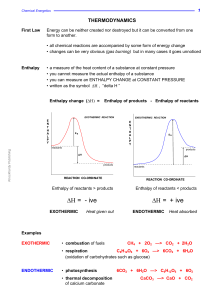
Physical and Chemical equilibrium
... Where K2 and K1 are equilibrium constant at temperature T2 and T1 respectively ∆HO is the enthalpy of reaction and R is the gas constant If ∆HO = 0, no heat is absorbed or evolved in the reaction ...
... Where K2 and K1 are equilibrium constant at temperature T2 and T1 respectively ∆HO is the enthalpy of reaction and R is the gas constant If ∆HO = 0, no heat is absorbed or evolved in the reaction ...
Document
... N2(g) + 3H2(g) 2NH3(g) For the reaction, H° = – 92.2 kJ and K (at 25°C) = 4.0 × 108. When the temperature of the reaction is increased to 500°C, which of the following is true? A) K for the reaction will be larger at 500°C than at 25°C. B) At equilibrium, more NH3 is present at 500°C than at 25°C. ...
... N2(g) + 3H2(g) 2NH3(g) For the reaction, H° = – 92.2 kJ and K (at 25°C) = 4.0 × 108. When the temperature of the reaction is increased to 500°C, which of the following is true? A) K for the reaction will be larger at 500°C than at 25°C. B) At equilibrium, more NH3 is present at 500°C than at 25°C. ...
1.24 calculations and chemical reactions
... Step 2 use balanced equation to work out which reactant is in excess Using 1TiCl4 :4 Na ratio we can see that 0.527mol of TiCl4 should react with 2.108 mol of Na. We actually have 3.48 mole of Na which is an excess of 1.372 moles. We can complete calculation using the limiting reactant of TiCl4 Step ...
... Step 2 use balanced equation to work out which reactant is in excess Using 1TiCl4 :4 Na ratio we can see that 0.527mol of TiCl4 should react with 2.108 mol of Na. We actually have 3.48 mole of Na which is an excess of 1.372 moles. We can complete calculation using the limiting reactant of TiCl4 Step ...
Chapter 20 Electrochemistry
... A strong oxidizing agent is one that readily gains electrons and thus has a large positive value of Eredo for its half-reaction. The larger the positive value of Eredo the greater is the tendency for the reduction half-reaction to occur. A strong oxidizing agent is one that readily gains electrons a ...
... A strong oxidizing agent is one that readily gains electrons and thus has a large positive value of Eredo for its half-reaction. The larger the positive value of Eredo the greater is the tendency for the reduction half-reaction to occur. A strong oxidizing agent is one that readily gains electrons a ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.