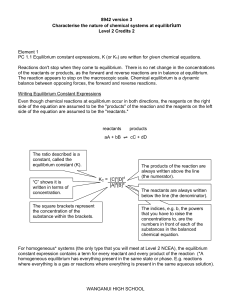
Chem 480A
... the reaction will proceed spontaneously as written and if rGo > the reaction will not proceed spontaneously as written (but the reverse reaction will proceed spontaneously). rGo can also be written in terms of chemical potentials of the components. rG o = cCo + dDo - aAo - bBo. ...
... the reaction will proceed spontaneously as written and if rGo > the reaction will not proceed spontaneously as written (but the reverse reaction will proceed spontaneously). rGo can also be written in terms of chemical potentials of the components. rG o = cCo + dDo - aAo - bBo. ...
free energy
... reaction) ΔG°) is the change in free energy under STANDARD CONDITIONS. It is related to the equilibrium constant by the equation: ΔG° = - RT ln Keq Where R : is the natural gas constant equal 8.315 joules or 1.987 Cal T :is the absolute temperature ln : natural logarithm. Keq : equilibrium constant ...
... reaction) ΔG°) is the change in free energy under STANDARD CONDITIONS. It is related to the equilibrium constant by the equation: ΔG° = - RT ln Keq Where R : is the natural gas constant equal 8.315 joules or 1.987 Cal T :is the absolute temperature ln : natural logarithm. Keq : equilibrium constant ...
Wanganui High School
... Reactions don't stop when they come to equilibrium. There is no net change in the concentrations of the reactants or products, as the forward and reverse reactions are in balance at equilibrium. The reaction appears to stop on the macroscopic scale. Chemical equilibrium is a dynamic balance between ...
... Reactions don't stop when they come to equilibrium. There is no net change in the concentrations of the reactants or products, as the forward and reverse reactions are in balance at equilibrium. The reaction appears to stop on the macroscopic scale. Chemical equilibrium is a dynamic balance between ...
General Equilibrium
... In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can be substituted for activities. (This assumption isn’t always good!) ...
... In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can be substituted for activities. (This assumption isn’t always good!) ...
1 ChE 505 WORKSHOP 1 1. Why are chemical reactions important
... Calculate the equilibrium conversion of SO2 reacting with air at 25˚C and at 600˚Cfor a feed that contains 1) stoichiometric ratio of reactants 2) large excess of air. Assume constant heat or reaction. ...
... Calculate the equilibrium conversion of SO2 reacting with air at 25˚C and at 600˚Cfor a feed that contains 1) stoichiometric ratio of reactants 2) large excess of air. Assume constant heat or reaction. ...
GENERAL CHEMISTRY II QUIZ 5 November 10, 2009
... 0.600 mol of O2 are placed in a 2.00 L flask at 600 K, what are the equilibrium concentrations of all species? N2(g) + ...
... 0.600 mol of O2 are placed in a 2.00 L flask at 600 K, what are the equilibrium concentrations of all species? N2(g) + ...
Unit 8: Equilibrium Content Outline: Shifting Equilibrium and Le
... II. Le Châtelier’s Principle A. This scientific observation was proposed by the French Chemist Henri Louis Le Châtelier in 1888. B. This principle is a method of predicting a “shift” in equilibrium for a chemical reaction. 1. The “shift” (Direction) is influenced by “stress factors”, such as concent ...
... II. Le Châtelier’s Principle A. This scientific observation was proposed by the French Chemist Henri Louis Le Châtelier in 1888. B. This principle is a method of predicting a “shift” in equilibrium for a chemical reaction. 1. The “shift” (Direction) is influenced by “stress factors”, such as concent ...
Chemical Equilibrium II
... Where ____ is known as the ___________ ___________ Note that the equilibrium expression can be expressed by concentrations in terms of _________ for aqueous solutions or _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to foll ...
... Where ____ is known as the ___________ ___________ Note that the equilibrium expression can be expressed by concentrations in terms of _________ for aqueous solutions or _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to foll ...
Le Chatelier`s Principle Quiz Answer Key
... If the statement is true, write "true"on your answer sheet. If it is false, change the underlined word or words to make the statement true and write the corrected answer on your answer sheet. NH4Cl(s) + heat NH3(g) + HCl(g) 5. The above reaction is exothermic. 6. The production of ammonia from amm ...
... If the statement is true, write "true"on your answer sheet. If it is false, change the underlined word or words to make the statement true and write the corrected answer on your answer sheet. NH4Cl(s) + heat NH3(g) + HCl(g) 5. The above reaction is exothermic. 6. The production of ammonia from amm ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.