Chemical Equations
... • Write the correct formulas for all reactants and products. • Determine the coefficients that make the equation balance. ...
... • Write the correct formulas for all reactants and products. • Determine the coefficients that make the equation balance. ...
Ionic Compounds 1. What is the formula for aluminum phosphate
... 2. A 87.2-g sample of SrCl2 is dissolved in 112.5 mL of solution. Calculate the molarity of this solution. 3. How many grams of NaCl are contained in 350. mL of a 0.171 M solution of sodium chloride? 4. What mass of calcium chloride, CaCl2, is in 3.576 L of a 1.56 M solution? 5. Which of the followi ...
... 2. A 87.2-g sample of SrCl2 is dissolved in 112.5 mL of solution. Calculate the molarity of this solution. 3. How many grams of NaCl are contained in 350. mL of a 0.171 M solution of sodium chloride? 4. What mass of calcium chloride, CaCl2, is in 3.576 L of a 1.56 M solution? 5. Which of the followi ...
The student will
... 1) Kinetics: Students analyze the reaction between bisulfite and iodate in a starch indicator to determine the order of the iodate in the reaction. They change concentration of the iodate ion and record the reaction time for the characteristic blue color to appear. Students also carry out the reacti ...
... 1) Kinetics: Students analyze the reaction between bisulfite and iodate in a starch indicator to determine the order of the iodate in the reaction. They change concentration of the iodate ion and record the reaction time for the characteristic blue color to appear. Students also carry out the reacti ...
Experiment 22
... decrease in reactant concentration causes a shift to the left; a decrease in product concentration produces a shift to the right. This is all true because Kc does not change (unless you change the temperature). The changes in concentration that one can produce by adding particular reagents may be si ...
... decrease in reactant concentration causes a shift to the left; a decrease in product concentration produces a shift to the right. This is all true because Kc does not change (unless you change the temperature). The changes in concentration that one can produce by adding particular reagents may be si ...
C4C5C6
... • Oil and water are immiscible - do not mix. • vegetable oil added to water + shaken well = emulsion. • An emulsion is one liquid finely dispersed in another • The shaking breaks up the oil into small droplets that disperse (spread out) in the water. – Milk is an oil-in-water emulsion that is mostly ...
... • Oil and water are immiscible - do not mix. • vegetable oil added to water + shaken well = emulsion. • An emulsion is one liquid finely dispersed in another • The shaking breaks up the oil into small droplets that disperse (spread out) in the water. – Milk is an oil-in-water emulsion that is mostly ...
2015 Academic Challenge CHEMISTRY TEST – STATE
... a solution that has too much solute for a given temperature. a mixture in which there is more solute than solvent. a solution in which the solvent has dissolved the maximum amount possible of a given solute at a given temperature. E. none of the above describes a saturated solution. ...
... a solution that has too much solute for a given temperature. a mixture in which there is more solute than solvent. a solution in which the solvent has dissolved the maximum amount possible of a given solute at a given temperature. E. none of the above describes a saturated solution. ...
H reactants
... 2. Changes in Concentration A + B C+D a. Increase in concentration of reactants – shift to producing more product (to use up reactant) Example: b. Decrease in concentration of reactants – shift to reverse reaction to make more of reactant Example ...
... 2. Changes in Concentration A + B C+D a. Increase in concentration of reactants – shift to producing more product (to use up reactant) Example: b. Decrease in concentration of reactants – shift to reverse reaction to make more of reactant Example ...
1 Chem 250 2nd Semester Exam Review Worksheet Part II
... 5. A sealed flask contains neon, argon, and krypton gas. If the total pressure in the flask is 3.782 atm, the partial pressure of Ne is 0.435 atm, and the partial pressure of Kr is 1.613 atm, what is the partial pressure of Ar in torr? ...
... 5. A sealed flask contains neon, argon, and krypton gas. If the total pressure in the flask is 3.782 atm, the partial pressure of Ne is 0.435 atm, and the partial pressure of Kr is 1.613 atm, what is the partial pressure of Ar in torr? ...
Word Equations • a summary
... From two days ago, the conservation of mass states: the total mass of the reactants = total mass of the products Why? In any chemical reaction, atoms are neither created nor destroyed, just rearranged. Therefore, because of the conservation of mass, chemical equations are balanced when the number of ...
... From two days ago, the conservation of mass states: the total mass of the reactants = total mass of the products Why? In any chemical reaction, atoms are neither created nor destroyed, just rearranged. Therefore, because of the conservation of mass, chemical equations are balanced when the number of ...
REACTION DYNAMICS
... What is the relationship between the potential energy surface for a chemical system and its reaction dynamics? How can quasi-classical trajectory (QCT) calculations be used in conjunction with experimental results to test the accuracy of a calculated potential energy surface? ...
... What is the relationship between the potential energy surface for a chemical system and its reaction dynamics? How can quasi-classical trajectory (QCT) calculations be used in conjunction with experimental results to test the accuracy of a calculated potential energy surface? ...
Chapter 5 - U of L Class Index
... Temperature. Raising the temperature will increase the number of collisions between molecules and also provide the collisions with the required energy of activation. Raising the temperature almost always increases the rate of reaction. Conversely, lowering the temperature will reduce the rate of rea ...
... Temperature. Raising the temperature will increase the number of collisions between molecules and also provide the collisions with the required energy of activation. Raising the temperature almost always increases the rate of reaction. Conversely, lowering the temperature will reduce the rate of rea ...
Chemistry 40S – Exam Review
... 2. Identify the conditions required for chemical equilibrium. 3. What statement is TRUE about a system at chemical equilibrium? a) observable changes occur during equilibrium b) the [ ]’s of reactants and products are equal c) the forward and reverse reaction rates are equal d) there are no reaction ...
... 2. Identify the conditions required for chemical equilibrium. 3. What statement is TRUE about a system at chemical equilibrium? a) observable changes occur during equilibrium b) the [ ]’s of reactants and products are equal c) the forward and reverse reaction rates are equal d) there are no reaction ...
Chemistry FINAL: CONTENT Review Packet
... _______________________is made from two or more substances that are physically combined ______________________________ are substances that are made up of only one type of atom _________________________________ is anything that has both mass and volume _____________________________________is a solid, ...
... _______________________is made from two or more substances that are physically combined ______________________________ are substances that are made up of only one type of atom _________________________________ is anything that has both mass and volume _____________________________________is a solid, ...
Atoms and Molecules
... I am very excited to have so many promising students sign-up for AP Chemistry. Often called the “central science”, chemistry is truly the best class you will ever take in high school. My goal is to prepare you for the AP exam, for college chemistry and for life as an informed member of our republic. ...
... I am very excited to have so many promising students sign-up for AP Chemistry. Often called the “central science”, chemistry is truly the best class you will ever take in high school. My goal is to prepare you for the AP exam, for college chemistry and for life as an informed member of our republic. ...
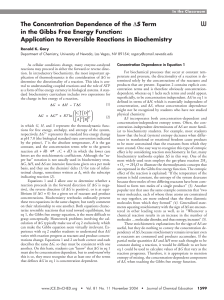
The Concentration Dependence of the
... to be more constrained than the reactants from which they were created. One easy way to recognize this type of entropic effect is by considering reaction stoichiometry. In fact, many biochemistry textbooks explain ∆S in this way. One of the most widely used texts employs the gas-phase reaction 2H2 + ...
... to be more constrained than the reactants from which they were created. One easy way to recognize this type of entropic effect is by considering reaction stoichiometry. In fact, many biochemistry textbooks explain ∆S in this way. One of the most widely used texts employs the gas-phase reaction 2H2 + ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.