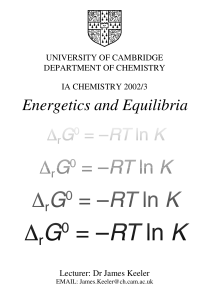Internal energy
... the enthalpy of evaporation drops to zero. Example: Enthalpy of evaporation of water is hLG = 2500 kJ/kg at 00C hLG = 2400 kJ/kg at 500C hLG = 2250 kJ/kg at 1000C hLG = 1900 kJ/kg at 2000C ...
... the enthalpy of evaporation drops to zero. Example: Enthalpy of evaporation of water is hLG = 2500 kJ/kg at 00C hLG = 2400 kJ/kg at 500C hLG = 2250 kJ/kg at 1000C hLG = 1900 kJ/kg at 2000C ...
Acid Base Equilibrium
... Note that the subscript “a” indicates that this is the equilibrium constant for the dissociation of an acid. • Note that [H2O] is omitted from the Ka expression. (H2O is a pure liquid.) The larger the Ka, the stronger the acid. • Ka is larger since there are more ions present at equilibrium relative ...
... Note that the subscript “a” indicates that this is the equilibrium constant for the dissociation of an acid. • Note that [H2O] is omitted from the Ka expression. (H2O is a pure liquid.) The larger the Ka, the stronger the acid. • Ka is larger since there are more ions present at equilibrium relative ...
Matter and Measurement
... In a chemical reaction, the enthalpy change during the reaction indicates whether the reaction releases energy or consumes energy. If DH < 0, the reaction releases heat and is EXOTHERMIC If DH > 0, the reaction absorbs heat and is ENDOTHERMIC ...
... In a chemical reaction, the enthalpy change during the reaction indicates whether the reaction releases energy or consumes energy. If DH < 0, the reaction releases heat and is EXOTHERMIC If DH > 0, the reaction absorbs heat and is ENDOTHERMIC ...
How to balance chemical equations.
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
Balancing Equations (A visual aid)
... I. Obtain a container of colored beads. If your container of beads does not have enough, get them from the reserve stockpile at the teacher’s desk. The numbers shown below are the minimum for you to be able to do the equation balancing. II. For equations (1) - (7) below, complete the following steps ...
... I. Obtain a container of colored beads. If your container of beads does not have enough, get them from the reserve stockpile at the teacher’s desk. The numbers shown below are the minimum for you to be able to do the equation balancing. II. For equations (1) - (7) below, complete the following steps ...
Aqueous chemistry is a very important component to laboratory
... overall reaction. The second is the ionic, where we focus on what is actually reacting (or not ...
... overall reaction. The second is the ionic, where we focus on what is actually reacting (or not ...
chemical reactions
... CHEMISTRY AND LIFE One unromantic but productive way of viewing life is to see it as a set of coordinated chemical reactions. ...
... CHEMISTRY AND LIFE One unromantic but productive way of viewing life is to see it as a set of coordinated chemical reactions. ...
SCH4U Exam Review
... 5. k = 64 for the reaction, N2 (g) + 3H2 (g) 2NH3 (g), at a certain temperature. Suppose it was found that an equilibrium mixture of these gases contained 0.280 M NH3 and 0.00840 M N2. What was the concentration of H2 in the mixture? ANS: 0.53 6. At high temperature, 0.500 mol of HBr was placed i ...
... 5. k = 64 for the reaction, N2 (g) + 3H2 (g) 2NH3 (g), at a certain temperature. Suppose it was found that an equilibrium mixture of these gases contained 0.280 M NH3 and 0.00840 M N2. What was the concentration of H2 in the mixture? ANS: 0.53 6. At high temperature, 0.500 mol of HBr was placed i ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.