Bohr-Rutherford Lewis Dot Diagrams Worksheet
... Draw the nucleus by first writing the symbol of the element and indicating the number of protons (p) and neutrons (n). Step 3: Draw the electrons in their orbits. Only a certain number of electrons can be held in each orbit: - fill the lower orbits (or energy levels) first - the first orbit will hol ...
... Draw the nucleus by first writing the symbol of the element and indicating the number of protons (p) and neutrons (n). Step 3: Draw the electrons in their orbits. Only a certain number of electrons can be held in each orbit: - fill the lower orbits (or energy levels) first - the first orbit will hol ...
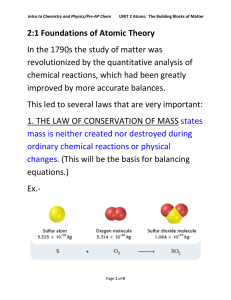
2:1 Foundations of Atomic Theory In the 1790s the study of matter
... and it is radioactive. It exists in very small amounts in nature but can be artificially prepared. ...
... and it is radioactive. It exists in very small amounts in nature but can be artificially prepared. ...
ViewpointAPBiology
... The number of protons in an atom determines the element – # of protons = atomic number – this also tells you # of electrons ...
... The number of protons in an atom determines the element – # of protons = atomic number – this also tells you # of electrons ...
Word List
... 3. Follow the link to “Groups of the Periodic Table” 4. When you get to the website, read and answer the following questions. 1. How are the families of the periodic table like real life families? 2. What is the definition of a metal? _________________________________________________________________ ...
... 3. Follow the link to “Groups of the Periodic Table” 4. When you get to the website, read and answer the following questions. 1. How are the families of the periodic table like real life families? 2. What is the definition of a metal? _________________________________________________________________ ...
Group 2 Elements
... • Also less attraction means electrons are pulled in less by the nucleus For each element the +2 ion is smaller than the atom because of the loss of the outer electrons. ...
... • Also less attraction means electrons are pulled in less by the nucleus For each element the +2 ion is smaller than the atom because of the loss of the outer electrons. ...
Particles in the Atom - IES Al
... elements pure substances because all atoms of an element were identical and that in particular they had the same mass. ...
... elements pure substances because all atoms of an element were identical and that in particular they had the same mass. ...
Atomic Theory - Hicksville Public Schools
... 2. Light emitted by excited electrons produces a distinct emission spectra for each element Valence Electrons - electrons in the outermost principal energy level a. The number of valence electrons directly relates to the chemical properties of an element 1. valence electrons match the group number i ...
... 2. Light emitted by excited electrons produces a distinct emission spectra for each element Valence Electrons - electrons in the outermost principal energy level a. The number of valence electrons directly relates to the chemical properties of an element 1. valence electrons match the group number i ...
chapter 2 - Columbia University
... LEUCIPUS of Miletus and his disciple DEMOCRITUS of Abdera: •Nature consists solely of an infinite number of indivisible particles, having shape, size, impenetrability, and no further properties. These particles move through an otherwise empty space. •The shape, size, location, and movement of these ...
... LEUCIPUS of Miletus and his disciple DEMOCRITUS of Abdera: •Nature consists solely of an infinite number of indivisible particles, having shape, size, impenetrability, and no further properties. These particles move through an otherwise empty space. •The shape, size, location, and movement of these ...
Interpreting Atomic Structure
... The gold foil experiment resulted in the discovery of the nucleus. According to Rutherford’s model, an atom was mostly open space. The “6+” in the model means that there are six protons in the nucleus. ...
... The gold foil experiment resulted in the discovery of the nucleus. According to Rutherford’s model, an atom was mostly open space. The “6+” in the model means that there are six protons in the nucleus. ...
Atomic Structure -
... The columns (groups or families) show the electron configuration is similar, so they have similar physical and chemical characteristics. The rows, or periods, are divided into 3 main areas: the metals (the largest), metalloids, and non-metals. Atomic # increases ...
... The columns (groups or families) show the electron configuration is similar, so they have similar physical and chemical characteristics. The rows, or periods, are divided into 3 main areas: the metals (the largest), metalloids, and non-metals. Atomic # increases ...
Isotopes - Cloudfront.net
... Across any period (horizontal row) the properties of elements gradually change – called a ...
... Across any period (horizontal row) the properties of elements gradually change – called a ...
Name
... . There is also a fourth and fifth phase,They are and , but they exist at very high temperatures. Science Is Fun Go to the “ChemTime Clock” area to find the answers to these questions. 1. All materials, whether solid, liquid or gas, are made of . Atoms are the smallest of . Scientists have found ove ...
... . There is also a fourth and fifth phase,They are and , but they exist at very high temperatures. Science Is Fun Go to the “ChemTime Clock” area to find the answers to these questions. 1. All materials, whether solid, liquid or gas, are made of . Atoms are the smallest of . Scientists have found ove ...
PAP Chemistry - Fall Final Review
... 8. Use the mass number and atomic number to determine the element and its number of protons, electrons, and neutrons 9. Be able to determine the atomic number and mass number of an element when the number of protons, neutrons, and electrons is specified 10. How does mass number relate to number of p ...
... 8. Use the mass number and atomic number to determine the element and its number of protons, electrons, and neutrons 9. Be able to determine the atomic number and mass number of an element when the number of protons, neutrons, and electrons is specified 10. How does mass number relate to number of p ...
PERIODIC TABLE OF THE ELEMENTS
... 4. Know how to write the Lewis Dot Structures for groups 1-2, & 3A8A(remember group 3A = group 13 and cetera). 5. Know how valence electrons and orbitals relate. 6. Know what orbitals are. 7. Know and understand elements and ions. 8. Know how to break a compound down into its ionic components using ...
... 4. Know how to write the Lewis Dot Structures for groups 1-2, & 3A8A(remember group 3A = group 13 and cetera). 5. Know how valence electrons and orbitals relate. 6. Know what orbitals are. 7. Know and understand elements and ions. 8. Know how to break a compound down into its ionic components using ...
Name: : ______ Chemistry—Matter, Atoms, and More Visit the
... _________________. Matter is anything that has a _________________. As of ___________ scientists have identified ___________________ states of matter. 12. You should know about ______________, _______________, _______________, and plasmas, and a new one called ________________-__________________ __ ...
... _________________. Matter is anything that has a _________________. As of ___________ scientists have identified ___________________ states of matter. 12. You should know about ______________, _______________, _______________, and plasmas, and a new one called ________________-__________________ __ ...
Lecture 2: Atoms - U of L Class Index
... Only a few elements have just one naturally occurring isotope (e.g. 19F, 23Na, 31P). Most elements occur as mixtures of several isotopes. Chemists normally treat these elements as consisting of “averaged” atoms with “averaged” masses. Atomic mass (as shown on the periodic table) is the weighted aver ...
... Only a few elements have just one naturally occurring isotope (e.g. 19F, 23Na, 31P). Most elements occur as mixtures of several isotopes. Chemists normally treat these elements as consisting of “averaged” atoms with “averaged” masses. Atomic mass (as shown on the periodic table) is the weighted aver ...
Electron Shells - rlas
... spin, they can move in any direction, as long as they stay in their _____________. Any direction you can imagine - upwards, downwards, or sideways - electrons can do it. The atomic shell, also called an _________________, is the distance from the nucleus that the electron spins. If you are an electr ...
... spin, they can move in any direction, as long as they stay in their _____________. Any direction you can imagine - upwards, downwards, or sideways - electrons can do it. The atomic shell, also called an _________________, is the distance from the nucleus that the electron spins. If you are an electr ...
Electron Configuration and Periodic Properties
... • Element A has a very low ionization energy, which means that atoms of A lose electrons easily. • Element A is most likely to be an s-block metal because ionization energies increase across the periods. • Element B has a very high ionization energy which means that atoms of B have difficulty losing ...
... • Element A has a very low ionization energy, which means that atoms of A lose electrons easily. • Element A is most likely to be an s-block metal because ionization energies increase across the periods. • Element B has a very high ionization energy which means that atoms of B have difficulty losing ...
Parts of the Atom - Issaquah Connect
... Parts of the Atom: 2 parts: Nucleus and electron cloud ________ ...
... Parts of the Atom: 2 parts: Nucleus and electron cloud ________ ...