FREE Sample Here
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. A ...
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. A ...
FREE Sample Here
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
FREE Sample Here
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. A ...
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. A ...
Biology, 8e (Campbell) Chapter 2 The Chemical Context of Life
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. ...
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. ...
- Deans Community High School
... b) Is the forward reaction is exothermic or endothermic. c) Gold and platinum both catalyse the reaction. For the forward reaction E A using gold is 30 kJ, while EA using platinum is 40 kJ. i) using different dotted lines add this information to the graph. ii) which is the better catalyst for the re ...
... b) Is the forward reaction is exothermic or endothermic. c) Gold and platinum both catalyse the reaction. For the forward reaction E A using gold is 30 kJ, while EA using platinum is 40 kJ. i) using different dotted lines add this information to the graph. ii) which is the better catalyst for the re ...
2.6 M - Thierry Karsenti
... If you get 6 items or more correct you can consider that you are doing fine, but if you get less than 4 items correct then you have to work very hard to pass the course. ...
... If you get 6 items or more correct you can consider that you are doing fine, but if you get less than 4 items correct then you have to work very hard to pass the course. ...
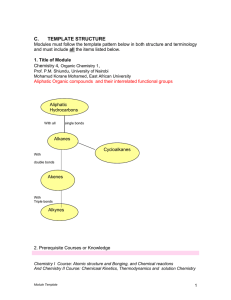
1 FORMATION OF THE ATOMIC THEORY
... requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his atomic theory, it attracted some attention. However, it failed to gain unanimous support. Some sup ...
... requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his atomic theory, it attracted some attention. However, it failed to gain unanimous support. Some sup ...
Biology, 8e (Campbell) Chapter 2 The Chemical Context of Life
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. ...
... E) There are covalent bonds between the hydrogen atoms. Answer: A Topic: Concept 2.3 Skill: Knowledge/Comprehension 46) When two atoms are equally electronegative, they will interact to form A) equal numbers of isotopes. B) ions. C) polar covalent bonds. D) nonpolar covalent bonds. E) ionic bonds. ...
File
... The mass of the products is always greater than the mass of the reactants. b. The mass of the reactants equals the mass of the products. c. The mass of the reactants increases. d. The mass of the products is always less than the mass of the reactants. ...
... The mass of the products is always greater than the mass of the reactants. b. The mass of the reactants equals the mass of the products. c. The mass of the reactants increases. d. The mass of the products is always less than the mass of the reactants. ...
Atomic structure and periodic table
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
Slide 2.1 - Cloudfront.net
... • All the chemical reactions in the body • Anabolism • Builds larger molecules • Ex: Combining many amino acids to form protein ...
... • All the chemical reactions in the body • Anabolism • Builds larger molecules • Ex: Combining many amino acids to form protein ...
Balancing Redox Reactions 1 - VCC Library
... In a redox reaction, the substance that gets oxidized (that loses electrons) is called the reducing agent because it reduces the other substance by giving its electrons. The substance that gets reduced (that gains electrons) is called the oxidizing agent because it oxidizes the other substance by re ...
... In a redox reaction, the substance that gets oxidized (that loses electrons) is called the reducing agent because it reduces the other substance by giving its electrons. The substance that gets reduced (that gains electrons) is called the oxidizing agent because it oxidizes the other substance by re ...
Covalent Bonding and Nomenclature
... How can you differentiate between a trigonal planar molecule and a trigonal pyramidal molecule in terms of nonbonding electron pairs on the central atom? Trigonal planar molecules do not have nonbonding electrons on the central atom. Trigonal pyramidal molecules have a pair of nonbonding electrons ...
... How can you differentiate between a trigonal planar molecule and a trigonal pyramidal molecule in terms of nonbonding electron pairs on the central atom? Trigonal planar molecules do not have nonbonding electrons on the central atom. Trigonal pyramidal molecules have a pair of nonbonding electrons ...
Final Exam Review 2010 UbD
... 40. What are the 2 conclusions Rutherford made about the structure of the atom after his Gold Foil Experiment? ___________________________________________________________________________ ______________________________________________________________________________________ 41. What is the mass of t ...
... 40. What are the 2 conclusions Rutherford made about the structure of the atom after his Gold Foil Experiment? ___________________________________________________________________________ ______________________________________________________________________________________ 41. What is the mass of t ...
Electrons in Atoms
... and magnetic fields are propagated as waves through empty space (a vacuum) or through a medium, such as glass. A wave is a disturbance that transmits energy through space or a material medium. Anyone who has sat in a small boat on a large body of water has experienced wave motion. The wave moves acr ...
... and magnetic fields are propagated as waves through empty space (a vacuum) or through a medium, such as glass. A wave is a disturbance that transmits energy through space or a material medium. Anyone who has sat in a small boat on a large body of water has experienced wave motion. The wave moves acr ...
Molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.