0922085
... be translated and adopted progressively (ECE/TRANS/WP.15/AC.2/30, paras. 38 and 40). ...
... be translated and adopted progressively (ECE/TRANS/WP.15/AC.2/30, paras. 38 and 40). ...
English Medium
... What are the questions do you ask? Prepare a model table to record the information that you have collected? 3. Some aged people will suffer while reading or writing due to their defect in vision i) Which lens do you suggest to them? ii) If that person can see only nearer objects and cannot see far o ...
... What are the questions do you ask? Prepare a model table to record the information that you have collected? 3. Some aged people will suffer while reading or writing due to their defect in vision i) Which lens do you suggest to them? ii) If that person can see only nearer objects and cannot see far o ...
CHEMISTRY 123-07 Midterm #1 – Answer key October 14, 2010
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
Chemical Equations and Reactions
... form definite molecular structures. Two exceptions to this rule are sulfur, which is usually written S8, and phosphorus, which is usually written P4. In these cases, the formulas reflect each element’s unique atomic arrangement in its natural state. 3. The law of conservation of mass must be satisfi ...
... form definite molecular structures. Two exceptions to this rule are sulfur, which is usually written S8, and phosphorus, which is usually written P4. In these cases, the formulas reflect each element’s unique atomic arrangement in its natural state. 3. The law of conservation of mass must be satisfi ...
AP Chemistry
... 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. products at a lower energy state than reactants (stronger bonds) 2. surroundings gain energy (heat up) 5. thermochemical equation a. chemical equation with H 1. li ...
... 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. products at a lower energy state than reactants (stronger bonds) 2. surroundings gain energy (heat up) 5. thermochemical equation a. chemical equation with H 1. li ...
Description: This is an advanced placement course designed to
... 4. Relationship of change in free energy to equilibrium constants and electrode potentials IV. Descriptive Chemistry (10-15%) Knowledge of specific facts of chemistry is essential for an understanding of principles and concepts. These descriptive facts, including the chemistry involved in environmen ...
... 4. Relationship of change in free energy to equilibrium constants and electrode potentials IV. Descriptive Chemistry (10-15%) Knowledge of specific facts of chemistry is essential for an understanding of principles and concepts. These descriptive facts, including the chemistry involved in environmen ...
File
... AP Thermodynamics Packet Unit 9 Thermodynamics Review Students should be able to demonstrate an understanding of the following essential knowledge: 3.C.2 Net changes in energy for a chemical reaction can be endothermic or exothermic. 5.A.1 Temperature is a measure of the average kinetic energy ...
... AP Thermodynamics Packet Unit 9 Thermodynamics Review Students should be able to demonstrate an understanding of the following essential knowledge: 3.C.2 Net changes in energy for a chemical reaction can be endothermic or exothermic. 5.A.1 Temperature is a measure of the average kinetic energy ...
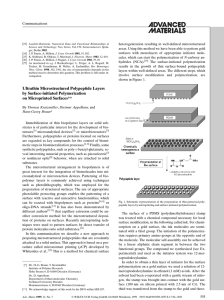
amcommu 555..558 - Leibniz-Institut für Polymerforschung Dresden
... leads to surface-bound poly(L-glutamic acid). This is a convenient route for the preparation of ultrathin layers of surface-bound polyelectrolytes. HBr in acetic acid was used for the hydrolysis of the benzylester group of poly-g-benzyl-glutamate. The hydrolysis of the benzylester group could also b ...
... leads to surface-bound poly(L-glutamic acid). This is a convenient route for the preparation of ultrathin layers of surface-bound polyelectrolytes. HBr in acetic acid was used for the hydrolysis of the benzylester group of poly-g-benzyl-glutamate. The hydrolysis of the benzylester group could also b ...
The s-Block Elements
... 2. For Group II sulphates, the cations are much smaller than the anions. The changing in size of cations does not cause a significant change in H lattice (proportional to 1/(r+ + r-). However, the changing in size of cations does cause H hydration (proportional to 1/r+ and 1/r-) to become less exo ...
... 2. For Group II sulphates, the cations are much smaller than the anions. The changing in size of cations does not cause a significant change in H lattice (proportional to 1/(r+ + r-). However, the changing in size of cations does cause H hydration (proportional to 1/r+ and 1/r-) to become less exo ...
Energetics Past Paper Questions
... State the name of the term ∆H˚. State, with a reason, whether reaction I would be accompanied by a decrease or increase in temperature. (3) At room temperature sulfur trioxide, SO3, is a solid. Deduce, with a reason, whether the ∆H˚ value would be more negative or less negative if SO3(s) instead of ...
... State the name of the term ∆H˚. State, with a reason, whether reaction I would be accompanied by a decrease or increase in temperature. (3) At room temperature sulfur trioxide, SO3, is a solid. Deduce, with a reason, whether the ∆H˚ value would be more negative or less negative if SO3(s) instead of ...
Organic Chemistry
... hydrocarbons undergo certain limited classes of reactions, many more reactions which organic compounds undergo are largely determined by functional groups. The general theory of these reactions involve careful analysis of properties such as the electron affinity of key atoms, bond strengths and ster ...
... hydrocarbons undergo certain limited classes of reactions, many more reactions which organic compounds undergo are largely determined by functional groups. The general theory of these reactions involve careful analysis of properties such as the electron affinity of key atoms, bond strengths and ster ...
Chemistry IGCSE
... Some substances never exist in a liquid form. If they are solid and you heat them they turn into a gas, and if they are a gas and you cool them they turn into a solid. This process is called Sublimation. The change in state occurs when the temperature is raised or dropped. Melting occurs when you he ...
... Some substances never exist in a liquid form. If they are solid and you heat them they turn into a gas, and if they are a gas and you cool them they turn into a solid. This process is called Sublimation. The change in state occurs when the temperature is raised or dropped. Melting occurs when you he ...
class xii – preparatory examination - 1
... [NiCl4]2- has tetrahedral geometry and paramagnetic.Explain these characteristics on the basis of hybridization of orbitals.[At no. of Ni=28] ii) Give an example of coordination compounds used in biological systems. 25. Complete the following reactions and balance them : i) NH3 + NaOCl ---- ii) P4O ...
... [NiCl4]2- has tetrahedral geometry and paramagnetic.Explain these characteristics on the basis of hybridization of orbitals.[At no. of Ni=28] ii) Give an example of coordination compounds used in biological systems. 25. Complete the following reactions and balance them : i) NH3 + NaOCl ---- ii) P4O ...
Chapter 9 Stoichiometry
... When baking cookies, a recipe is usually used, telling the exact amount of each ingredient ...
... When baking cookies, a recipe is usually used, telling the exact amount of each ingredient ...
Chemistry Revision Checklist F4 2017 (inc F3)
... Describe the general characteristics of an homologous series Recall that the compounds in a homologous series have the same general formula Describe and identify structural isomerism Describe the properties of alkanes (exemplified by methane) as being generally unreactive, except in terms of burning ...
... Describe the general characteristics of an homologous series Recall that the compounds in a homologous series have the same general formula Describe and identify structural isomerism Describe the properties of alkanes (exemplified by methane) as being generally unreactive, except in terms of burning ...
The Major Classes of Chemical Reactions
... how water acts as a solvent. The role a solvent plays in a reaction depends on its chemical nature. Some solvents play a passive role. They disperse the substances into individual molecules but do not interact with them in other ways. Water plays a much more active role. It interacts strongly with t ...
... how water acts as a solvent. The role a solvent plays in a reaction depends on its chemical nature. Some solvents play a passive role. They disperse the substances into individual molecules but do not interact with them in other ways. Water plays a much more active role. It interacts strongly with t ...
The d block:
... Chromium and Copper • Cr and Cu don’t fit the pattern of building up the 3d sub-shell, why? – In the ground state electrons are always arranged to give lowest total energy – Electrons are negatively charged and repel each other – Lower total energy is obtained with e- singly in orbitals rather than ...
... Chromium and Copper • Cr and Cu don’t fit the pattern of building up the 3d sub-shell, why? – In the ground state electrons are always arranged to give lowest total energy – Electrons are negatively charged and repel each other – Lower total energy is obtained with e- singly in orbitals rather than ...
Oxidation numbers
... fact that many Transition Metals are characterized by a partially filled inner electron level, inside the valence shell. Electrons within this inner shell may sometimes behave as valence electrons and are lost along with the outermost electrons during oxidation. The number of electrons lost depends ...
... fact that many Transition Metals are characterized by a partially filled inner electron level, inside the valence shell. Electrons within this inner shell may sometimes behave as valence electrons and are lost along with the outermost electrons during oxidation. The number of electrons lost depends ...
SOLUBILITY RULES FOR IONIC COMPOUNDS IN WATER
... (a) An acidified solution of hydrogen peroxide is added to a solution of sodium iodide. (b) Chlorine gas is passed over powdered aluminum. (c) Solutons of mercury (I) nitrate and potassium sulfate are mixed. (d) A strip of magnesium metal is added to a solution of silver nitrate. (e) Solutions of le ...
... (a) An acidified solution of hydrogen peroxide is added to a solution of sodium iodide. (b) Chlorine gas is passed over powdered aluminum. (c) Solutons of mercury (I) nitrate and potassium sulfate are mixed. (d) A strip of magnesium metal is added to a solution of silver nitrate. (e) Solutions of le ...
chapter 13 - Humble ISD
... We replace NaOH with X and H3PO4 with Y we get: 3X=Y Let’s look at the equation now: Keq = [0.200] ...
... We replace NaOH with X and H3PO4 with Y we get: 3X=Y Let’s look at the equation now: Keq = [0.200] ...
CHAPTER 15 ACIDS AND BASES
... At pH 1.00 the concentration of hydrogen ion is 0.10 M (Why only two significant figures?) This will tend to suppress the ionization of the weak acid (LeChatelier's principle, Section 14.5). The extra hydrogen ion shifts the position of equilibrium in the direction of the un-ionized acid, and to two ...
... At pH 1.00 the concentration of hydrogen ion is 0.10 M (Why only two significant figures?) This will tend to suppress the ionization of the weak acid (LeChatelier's principle, Section 14.5). The extra hydrogen ion shifts the position of equilibrium in the direction of the un-ionized acid, and to two ...
Tall: 1) The decomposition of CaCO3 is an endothermic process:
... A 1.00 mol sample of CO2 is heated to 1000K with excess graphite in a container of volume 40.0 L. At this temperature, Kc is 2.11x10-2 for the reaction: C(graphite) + CO2(g) 2 CO(g) a) b) ...
... A 1.00 mol sample of CO2 is heated to 1000K with excess graphite in a container of volume 40.0 L. At this temperature, Kc is 2.11x10-2 for the reaction: C(graphite) + CO2(g) 2 CO(g) a) b) ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.