Prospective Chemistry Teachers` Conceptions of Chemical
... conceptual difficulties in making transformation within and across different theoretical models indicating that they were not able to use scientifically acceptable concepts of reaction rate across context and displayed misconceptions. Purpose and Research Question Although several studies cited abov ...
... conceptual difficulties in making transformation within and across different theoretical models indicating that they were not able to use scientifically acceptable concepts of reaction rate across context and displayed misconceptions. Purpose and Research Question Although several studies cited abov ...
Separation and Purification Methods
... pressure, or vacuum distillation, brings many advantages. For example, consider a liquid that has a boiling point of 180◦ C at atmospheric pressure (760 Torr). If you were to attempt that distillation, you would not be surprised to observe charring of the compound at such elevated temperature. Fortu ...
... pressure, or vacuum distillation, brings many advantages. For example, consider a liquid that has a boiling point of 180◦ C at atmospheric pressure (760 Torr). If you were to attempt that distillation, you would not be surprised to observe charring of the compound at such elevated temperature. Fortu ...
Thermodynamics - Shailendra Kumar Chemistry
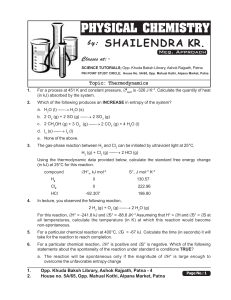
... For particular chemical reaction, both ∆H° and ∆S° are negative. Which of the following statements about the spontaneity of the reaction under standard conditions is TRUE? a. The reaction will be spontaneous only if the magnitude of ∆H° is large enough to overcome the unfavorable entropy change. b. ...
... For particular chemical reaction, both ∆H° and ∆S° are negative. Which of the following statements about the spontaneity of the reaction under standard conditions is TRUE? a. The reaction will be spontaneous only if the magnitude of ∆H° is large enough to overcome the unfavorable entropy change. b. ...
chemistry intermediate may 2010 marking scheme
... (i) What volume of hydrogen chloride, in dm3 measured at s.t.p. can be obtained by the action of excess concentrated sulfuric acid on 11.70g sodium chloride? 11.7 g NaCl contains 11.7/[23+35.5] = 0.2 mol (1) Mol HCl produced = 0.2; (1) Volume of gas at stp = 0.2X 22.4 dm3 (1) ...
... (i) What volume of hydrogen chloride, in dm3 measured at s.t.p. can be obtained by the action of excess concentrated sulfuric acid on 11.70g sodium chloride? 11.7 g NaCl contains 11.7/[23+35.5] = 0.2 mol (1) Mol HCl produced = 0.2; (1) Volume of gas at stp = 0.2X 22.4 dm3 (1) ...
Solution of the 1st Major Exam, Term 061, Version 000, all correct
... CrO42-(aq) + Cl-(aq) Cr3+(aq) + ClO2-(aq) is balanced with the smallest possible set of integer numbers, the coefficient of CrO42- will be (the reaction takes place in acidic medium): A) 4 ...
... CrO42-(aq) + Cl-(aq) Cr3+(aq) + ClO2-(aq) is balanced with the smallest possible set of integer numbers, the coefficient of CrO42- will be (the reaction takes place in acidic medium): A) 4 ...
Copy of Acids, bases, salts answer key
... Potassium hydroxide Potassium metal ion Hydroxide ion Limitations of Arrhenius theory : Arhhenius’ theory became quite popular and was widely accepted yet it had the following limitations: This theory was applicable only to aqueous solutions. Substances like Ammonia (NH3) do not contain hydroxid ...
... Potassium hydroxide Potassium metal ion Hydroxide ion Limitations of Arrhenius theory : Arhhenius’ theory became quite popular and was widely accepted yet it had the following limitations: This theory was applicable only to aqueous solutions. Substances like Ammonia (NH3) do not contain hydroxid ...
PDF Chapter 14 Chemical Kinetics
... Gasoline and air in a car engine explode violently, but left untouched, they will not react for years at a time. Meat left out will invite biochemical reactions that, among other thing, generate bad smelling gases. If kept at lower temperatures, these reactions take a much longer time to occur. En ...
... Gasoline and air in a car engine explode violently, but left untouched, they will not react for years at a time. Meat left out will invite biochemical reactions that, among other thing, generate bad smelling gases. If kept at lower temperatures, these reactions take a much longer time to occur. En ...
Belarus, National Final, 2008 (PDF 405K).
... a) How would you prepare 5 salts containing iron and 5 salts containing aluminum? Write balanced chemical equations for the preparation of these 10 salts and specify conditions under which these reactions occur. b) Give an example of a salt of iron in which iron is found in the highest possible oxid ...
... a) How would you prepare 5 salts containing iron and 5 salts containing aluminum? Write balanced chemical equations for the preparation of these 10 salts and specify conditions under which these reactions occur. b) Give an example of a salt of iron in which iron is found in the highest possible oxid ...
WELCOME TO CLASS XII ORIENTATION IN CHEMISTRY SOME
... adding a strong electropositive metal like ...
... adding a strong electropositive metal like ...
5 SURFACE CHEMISTRY CATEGORY
... 3.Define the term osmotic pressure. Describe how the molecular mass of a substance can be determined by a method based on measurement of osmotic pressure? 4.Define osmotic pressure. How is it that measurement of osmotic pressures is more widely used for determining molar masses of macromolecules tha ...
... 3.Define the term osmotic pressure. Describe how the molecular mass of a substance can be determined by a method based on measurement of osmotic pressure? 4.Define osmotic pressure. How is it that measurement of osmotic pressures is more widely used for determining molar masses of macromolecules tha ...
Reactions and Stoichiometry Practice Problems
... 1) Which of the following is an example of a physical change? A) burning coal B) converting water to hydrogen and oxygen C) baking a cake D) digesting a cheeseburger E) grinding coffee beans ...
... 1) Which of the following is an example of a physical change? A) burning coal B) converting water to hydrogen and oxygen C) baking a cake D) digesting a cheeseburger E) grinding coffee beans ...
2006 Practice Final Exam - Department of Chemistry | Oregon State
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
Part II - KFUPM Faculty List
... Thermodynamics of Living Systems Thermodynamics have a great effect in biological sciences, such as processes taking place inside our bodies. such as processes taking place inside our bodies. Many chemical reactions carried out inside the body (such as DNA and protein formation) are not sponta ...
... Thermodynamics of Living Systems Thermodynamics have a great effect in biological sciences, such as processes taking place inside our bodies. such as processes taking place inside our bodies. Many chemical reactions carried out inside the body (such as DNA and protein formation) are not sponta ...
North Carolina Test of Chemistry RELEASED
... written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
... written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
chapter4-bur.2917051..
... 2) Atoms in elemental forms have an oxidation number of 0. 3) Hydrogen has an oxidation number of +1 when bonded with nonmetals (molecular compounds) and -1 when combined with metals (ionic compounds). ...
... 2) Atoms in elemental forms have an oxidation number of 0. 3) Hydrogen has an oxidation number of +1 when bonded with nonmetals (molecular compounds) and -1 when combined with metals (ionic compounds). ...
Part-1
... if bigger, then octahedral voids. Not all octrahedral or teterahedral voids are occupied. The fraction of octahedral or tetrahedral voids that are occupied, depends upon the chemical formula of the ...
... if bigger, then octahedral voids. Not all octrahedral or teterahedral voids are occupied. The fraction of octahedral or tetrahedral voids that are occupied, depends upon the chemical formula of the ...
Exam - Vcaa
... The equation for the redox reaction is 2CuO(s) 2Cu(s) + O2(g) The gas passing through the tube prevented the copper from re-oxidising to CuO. The students weighed: • the empty tube • the tube and CuO before heating • the tube and Cu after heating and cooling. They found that the percentage by mass ...
... The equation for the redox reaction is 2CuO(s) 2Cu(s) + O2(g) The gas passing through the tube prevented the copper from re-oxidising to CuO. The students weighed: • the empty tube • the tube and CuO before heating • the tube and Cu after heating and cooling. They found that the percentage by mass ...
No Slide Title
... 2) Atoms in elemental forms have an oxidation number of 0. 3) Hydrogen has an oxidation number of +1 when bonded with nonmetals (molecular compounds) and -1 when combined with metals (ionic compounds). ...
... 2) Atoms in elemental forms have an oxidation number of 0. 3) Hydrogen has an oxidation number of +1 when bonded with nonmetals (molecular compounds) and -1 when combined with metals (ionic compounds). ...
carbon compounds - Badhan Education
... Paraffins. It means little affinity or reactivity. Alkanes are also called paraffins because they have little affinity or reactivity. General Formula or Generic Formula. The formula from which each and every member of a specific family of organic compounds can be derived e. g., CnH2n + 2 is general ...
... Paraffins. It means little affinity or reactivity. Alkanes are also called paraffins because they have little affinity or reactivity. General Formula or Generic Formula. The formula from which each and every member of a specific family of organic compounds can be derived e. g., CnH2n + 2 is general ...
Skill Practice 1
... 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allows you to tell? 4. Assuming that the temperature scales for both phase diagrams are the same, which c ...
... 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allows you to tell? 4. Assuming that the temperature scales for both phase diagrams are the same, which c ...
Step by Step Stoichiometry
... These problems will ask you to calculate the amount of one substance, in grams, that will be needed to react or be produced from a given mass for another substance. The plan for solving these kinds of problems: Mass of given amount of given in moles amount of unknown in moles mass of ...
... These problems will ask you to calculate the amount of one substance, in grams, that will be needed to react or be produced from a given mass for another substance. The plan for solving these kinds of problems: Mass of given amount of given in moles amount of unknown in moles mass of ...
Equations - Pearson Schools and FE Colleges
... The equation is / is not balanced because there are the same number / different numbers of atoms of each element on each side of the equation. ...
... The equation is / is not balanced because there are the same number / different numbers of atoms of each element on each side of the equation. ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.