teaching and learning materials - UNESDOC
... experiences and low-cost equipment designs, has some limitations. In chemistry, for example, major areas of the subject have been ignored. One of these is organic chemistry. In several countries, the welcome accorded to the basic ideas, has been coupled with questions about these important missing a ...
... experiences and low-cost equipment designs, has some limitations. In chemistry, for example, major areas of the subject have been ignored. One of these is organic chemistry. In several countries, the welcome accorded to the basic ideas, has been coupled with questions about these important missing a ...
Organic Chemistry/Fourth Edition: e-Text
... The amino acids in Table 27.1 that have more than one stereogenic center are isoleucine and threonine. The stereogenic centers are marked with an asterisk in the structural formulas shown. ...
... The amino acids in Table 27.1 that have more than one stereogenic center are isoleucine and threonine. The stereogenic centers are marked with an asterisk in the structural formulas shown. ...
EQUILIBRIUM
... • 1st pt. → initial pH must be > 7 (calculated pH ≈ 11) • 2nd pt. → equivalence point occurs at 15.0 mL ± 1 mL of HCl added (equivalence point must be detectable from the shape of the curve or a mark on the curve) • 3rd pt. → pH at equivalence point must be < 7 (calculated pH ≈ 5). Note: a maximum o ...
... • 1st pt. → initial pH must be > 7 (calculated pH ≈ 11) • 2nd pt. → equivalence point occurs at 15.0 mL ± 1 mL of HCl added (equivalence point must be detectable from the shape of the curve or a mark on the curve) • 3rd pt. → pH at equivalence point must be < 7 (calculated pH ≈ 5). Note: a maximum o ...
AS Specification pdf | AS/A level
... industrial and environmental contexts. It is a requirement of all AS specifications that learners must demonstrate a holistic understanding of the links between different areas of content. In practice this means that questions set in any component may require learners to draw upon knowledge from oth ...
... industrial and environmental contexts. It is a requirement of all AS specifications that learners must demonstrate a holistic understanding of the links between different areas of content. In practice this means that questions set in any component may require learners to draw upon knowledge from oth ...
s-BLOCK ELEMENTS - einstein classes
... The properties of Group 2 (Alkaline earth metals) : (i) Density : The densities of alkaline earth metals are low but greater than alkali metals. The densities decreases from Be to Ca with the increase of atomic number and then increases from Sr to Ba. (ii) Melting point : Alkaline earth metals have ...
... The properties of Group 2 (Alkaline earth metals) : (i) Density : The densities of alkaline earth metals are low but greater than alkali metals. The densities decreases from Be to Ca with the increase of atomic number and then increases from Sr to Ba. (ii) Melting point : Alkaline earth metals have ...
+ 2 H2O(l Ca(OH)2 aq)
... c) 2 KMnO4(aq) + 3 Na2SO3(aq) + H2O(l) 2 MnO2(s) + 3 Na2SO4(aq) + 2 KOH(aq) KMnO4 is the oxidizing agent (O.N.(Mn) goes from +7 to +4). Na2SO3 is the reducing agent (O.N.(S) goes from +4 to +6). d) 2 CrO42–(aq) + 3 HSnO2–(aq) + H2O(l) 2 CrO2–(aq) + 3 HSnO3–(aq) + 2 OH–(aq) CrO42– is the oxidizin ...
... c) 2 KMnO4(aq) + 3 Na2SO3(aq) + H2O(l) 2 MnO2(s) + 3 Na2SO4(aq) + 2 KOH(aq) KMnO4 is the oxidizing agent (O.N.(Mn) goes from +7 to +4). Na2SO3 is the reducing agent (O.N.(S) goes from +4 to +6). d) 2 CrO42–(aq) + 3 HSnO2–(aq) + H2O(l) 2 CrO2–(aq) + 3 HSnO3–(aq) + 2 OH–(aq) CrO42– is the oxidizin ...
Organic Molecules
... In organic chemistry, a functional group is a speci c group of atoms within molecules, that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a par ...
... In organic chemistry, a functional group is a speci c group of atoms within molecules, that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a par ...
Summer Study Assignment – Honors Chem 2/AP Chemistry
... 31. Write the electron configuration using the Noble Gas core method for californium. 32. Write a balanced equation for the following double replacement reactions: a. Calcium hydroxide (aq) + nitric acid (aq) b. Chromium (III) sulfite (aq) + sulfuric acid (aq) c. Zinc chloride (aq) + ammonium su ...
... 31. Write the electron configuration using the Noble Gas core method for californium. 32. Write a balanced equation for the following double replacement reactions: a. Calcium hydroxide (aq) + nitric acid (aq) b. Chromium (III) sulfite (aq) + sulfuric acid (aq) c. Zinc chloride (aq) + ammonium su ...
Gas Laws
... Hydrogen is the only element that has an exposed proton when an electron is lost. The exposure of the proton and the fact that the other element that the hydrogen in bonded to has a very high electron affinity, the compound ends up having a very strong dipole moment called hydrogen bonding. 7. What ...
... Hydrogen is the only element that has an exposed proton when an electron is lost. The exposure of the proton and the fact that the other element that the hydrogen in bonded to has a very high electron affinity, the compound ends up having a very strong dipole moment called hydrogen bonding. 7. What ...
Chemistry - Separation techniques
... C3.1c use the names and symbols of common elements from a supplied periodic table to write formulae and balanced chemical equations where appropriate C3.1e construct balanced ionic equations C3.1f describe the physical states of products and reactants using state symbols (s, l, g and aq) C3.1k dedu ...
... C3.1c use the names and symbols of common elements from a supplied periodic table to write formulae and balanced chemical equations where appropriate C3.1e construct balanced ionic equations C3.1f describe the physical states of products and reactants using state symbols (s, l, g and aq) C3.1k dedu ...
Solutes
... Polar molecules dissolve other polar molecules and ionic compounds. and alcohols Nonpolar molecules dissolve other nonpolar molecules. and alcohols Alcohols, which have characteristics of both polar & nonpolar, tend to dissolve in both types of solvents, but will not dissolve ionic solids. and other ...
... Polar molecules dissolve other polar molecules and ionic compounds. and alcohols Nonpolar molecules dissolve other nonpolar molecules. and alcohols Alcohols, which have characteristics of both polar & nonpolar, tend to dissolve in both types of solvents, but will not dissolve ionic solids. and other ...
P. Mignon, J. Steyaert, R. Loris, P. Geerlings, and S. Loverix, J. Biol
... been shown to take part in catalysis (5). Both the His-40 and Glu-58 side chains are in the direct vicinity of the 2⬘-nucleophile. pH dependence studies have shown Glu-58 to be unprotonated and His-40 to be protonated at the onset of catalysis, proving that Glu-58 is the catalytic base accepting a p ...
... been shown to take part in catalysis (5). Both the His-40 and Glu-58 side chains are in the direct vicinity of the 2⬘-nucleophile. pH dependence studies have shown Glu-58 to be unprotonated and His-40 to be protonated at the onset of catalysis, proving that Glu-58 is the catalytic base accepting a p ...
Pirogov National Medical Univercity of Vinnitsa
... subject of work, will learn theoretical material with the help of textbooks, manuals and lecture notes, record the equations of respective reactions in the laboratory magazine. 2. Duty student receives the necessary work for the group equipment and reagents, and places them in the workplace. 3. In t ...
... subject of work, will learn theoretical material with the help of textbooks, manuals and lecture notes, record the equations of respective reactions in the laboratory magazine. 2. Duty student receives the necessary work for the group equipment and reagents, and places them in the workplace. 3. In t ...
Experiment 7 - MASSIVE REACTIONS
... Teachers each have various budgets and classroom situations to consider. Having students go into the laboratory and perform chemical reactions might be prohibitive because of cost factors, disposal concerns or an unproductive use of classroom time. An alternative approach that can provide students t ...
... Teachers each have various budgets and classroom situations to consider. Having students go into the laboratory and perform chemical reactions might be prohibitive because of cost factors, disposal concerns or an unproductive use of classroom time. An alternative approach that can provide students t ...
Example of Lab Notebook
... crystallization in an ice-water bath, the product underwent vacuum filtration to isolate. The product was dried and the purity of the product was assessed by running a melting point and by comparing the experimental value to the true value. To an acidic aqueous solution, 5.2 mL of aniline was added ...
... crystallization in an ice-water bath, the product underwent vacuum filtration to isolate. The product was dried and the purity of the product was assessed by running a melting point and by comparing the experimental value to the true value. To an acidic aqueous solution, 5.2 mL of aniline was added ...
Oxidation-Reduction Reactions
... Many elements simply combine with oxygen to form the oxide of that element. Heating magnesium in air allows it to combine with oxygen to form magnesium oxide. 2 Mg(s) + O2 (g) → 2MgO(s) Many compounds react with oxygen as well, often in very exothermic processes that are generally referred to as com ...
... Many elements simply combine with oxygen to form the oxide of that element. Heating magnesium in air allows it to combine with oxygen to form magnesium oxide. 2 Mg(s) + O2 (g) → 2MgO(s) Many compounds react with oxygen as well, often in very exothermic processes that are generally referred to as com ...
A flask contains 0
... If you have time after you did the starred () questions, go to the circle questions and look at them again…maybe a second time through will jog your memory. REMEMBER every time you turn the page…make sure that you have bubbled the correct number on the answer sheet. This way if you get off trac ...
... If you have time after you did the starred () questions, go to the circle questions and look at them again…maybe a second time through will jog your memory. REMEMBER every time you turn the page…make sure that you have bubbled the correct number on the answer sheet. This way if you get off trac ...
Isomers and Isomerism Isomers
... associatedthree-dimensional properties, molecules can have chirality as one stereochemical feature. Any object is chiral if it is different (nonsuperimposable) than its mirror image. Likewise a molecule is chiral if it is nonsuperimposable on its mirror image. This requirement does not consider conf ...
... associatedthree-dimensional properties, molecules can have chirality as one stereochemical feature. Any object is chiral if it is different (nonsuperimposable) than its mirror image. Likewise a molecule is chiral if it is nonsuperimposable on its mirror image. This requirement does not consider conf ...
AP® Chemistry
... Directions: Each set of lettered choices below refers to the numbered statements immediately following it. Select the one that is best in each case and then place the letter of your choice in the corresponding box on the student answer sheet. A choice may be used once, more than once, or not at all ...
... Directions: Each set of lettered choices below refers to the numbered statements immediately following it. Select the one that is best in each case and then place the letter of your choice in the corresponding box on the student answer sheet. A choice may be used once, more than once, or not at all ...
AP® Chemistry
... Directions: Each set of lettered choices below refers to the numbered statements immediately following it. Select the one that is best in each case and then place the letter of your choice in the corresponding box on the student answer sheet. A choice may be used once, more than once, or not at all ...
... Directions: Each set of lettered choices below refers to the numbered statements immediately following it. Select the one that is best in each case and then place the letter of your choice in the corresponding box on the student answer sheet. A choice may be used once, more than once, or not at all ...
PDF File
... ions, referred to as MA. We identified the pro-SP oxygen atoms at nucleotides C208, A304, and A306 as ground state ligands for MA, verifying interactions suggested by the Azoarcus crystal structures. We further established that these interactions are present in the chemical transition state, a concl ...
... ions, referred to as MA. We identified the pro-SP oxygen atoms at nucleotides C208, A304, and A306 as ground state ligands for MA, verifying interactions suggested by the Azoarcus crystal structures. We further established that these interactions are present in the chemical transition state, a concl ...
Use the following answers for questions 1
... (B) Carbon dioxide (C) Aluminum hydroxide (D) Ammonia (E) Hydrogen peroxide 4. Is a good oxidizing agent 5. Is used to etch glass chemically 6. Is used extensively for the production of fertilizers 7. Has amphoteric properties 63. Which of the following characteristics is common to elemental sulfur, ...
... (B) Carbon dioxide (C) Aluminum hydroxide (D) Ammonia (E) Hydrogen peroxide 4. Is a good oxidizing agent 5. Is used to etch glass chemically 6. Is used extensively for the production of fertilizers 7. Has amphoteric properties 63. Which of the following characteristics is common to elemental sulfur, ...
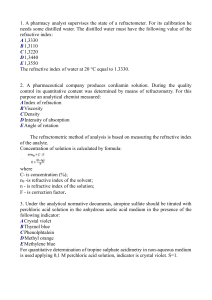
1. A pharmacy analyst supervises the state of a refractometer. For its
... required accuracy is almost impossible. To enhance the acidic properties of boric acid, it is reacted with polyatomic alcohols to form complexes having stronger acidic properties than boric acid itself. 19. In order to identify a drug an analytical chemist of the State Inspectorate for Quality Contr ...
... required accuracy is almost impossible. To enhance the acidic properties of boric acid, it is reacted with polyatomic alcohols to form complexes having stronger acidic properties than boric acid itself. 19. In order to identify a drug an analytical chemist of the State Inspectorate for Quality Contr ...
KISS Notes
... Chemically, the extraction of metals from ores involves aj)............................... reactions, which are ak).............-thermic. Some metals, such as al)............................. require very little energy, others such as am)...................................... require much more. In m ...
... Chemically, the extraction of metals from ores involves aj)............................... reactions, which are ak).............-thermic. Some metals, such as al)............................. require very little energy, others such as am)...................................... require much more. In m ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.