WJEC Eduqas A Level Chemistry specification
... This section outlines the knowledge, understanding and skills to be developed by learners studying A level Chemistry. Learners should be prepared to apply the knowledge, understanding and skills specified in a range of theoretical, practical, industrial and environmental contexts. It is a requiremen ...
... This section outlines the knowledge, understanding and skills to be developed by learners studying A level Chemistry. Learners should be prepared to apply the knowledge, understanding and skills specified in a range of theoretical, practical, industrial and environmental contexts. It is a requiremen ...
11.1 Enthalpy PowerPoint
... Yes, but indirectly. We can measure a change in temperature, we can then calculate the change in thermal energy (Q=mct). Then, using the law of conservation of energy we can infer the molar enthalpy. ...
... Yes, but indirectly. We can measure a change in temperature, we can then calculate the change in thermal energy (Q=mct). Then, using the law of conservation of energy we can infer the molar enthalpy. ...
Chemical Reactions Q3U3
... Experimentation has proved that, in some cases, not all of the reactants are converted to the product, no matter how much time is given. These reactions are reversible! ...
... Experimentation has proved that, in some cases, not all of the reactants are converted to the product, no matter how much time is given. These reactions are reversible! ...
([Cu(NH3)4](MnO4)2)
... The appearance of IR-inactive Cu N stretching modes in the IR spectrum of 1 shows the symmetry-lowering due to distortion of the regular square-planar CuN4 geometry. The splittings of nÄs , ds, and 1r N H bands or the nÄas Cu N band confirm the symmetry-lowering of the complex cation. The presence o ...
... The appearance of IR-inactive Cu N stretching modes in the IR spectrum of 1 shows the symmetry-lowering due to distortion of the regular square-planar CuN4 geometry. The splittings of nÄs , ds, and 1r N H bands or the nÄas Cu N band confirm the symmetry-lowering of the complex cation. The presence o ...
Catalytic decomposition of N2O over Rh/Zn–Al2O3 catalysts
... lead to more active catalysts due to the improved dispersion of Rh species.28 Parres-Esclapez et al. found that Sr can promote the activity of Rh/Al2O3 due to the improved dispersion and reducibility of Rh species.29 Zhao et al. reported that Rh/SiO2–Al2O3 shows high activity, because oxygen desorpt ...
... lead to more active catalysts due to the improved dispersion of Rh species.28 Parres-Esclapez et al. found that Sr can promote the activity of Rh/Al2O3 due to the improved dispersion and reducibility of Rh species.29 Zhao et al. reported that Rh/SiO2–Al2O3 shows high activity, because oxygen desorpt ...
biomolecules (introduction, structure
... fundamental structural and functional components of the body. They are nitrogenous “macromolecules” composed of many amino acids. Most proteins contain, in varying proportions, the same 20 L-α-amino acids. Many specific proteins contain, in addition, L-α-amino acids derived from some of the basic 20 ...
... fundamental structural and functional components of the body. They are nitrogenous “macromolecules” composed of many amino acids. Most proteins contain, in varying proportions, the same 20 L-α-amino acids. Many specific proteins contain, in addition, L-α-amino acids derived from some of the basic 20 ...
Chapter 4 - AP Chemistry with dr hart
... oxidation numbers, although some are positive in certain compounds or ions. Fluorine always has an oxidation number of −1. The other halogens have an oxidation number of −1 when they are negative; they can have positive oxidation numbers, Aqueous however, most notably in oxyanions. Reactions © 200 ...
... oxidation numbers, although some are positive in certain compounds or ions. Fluorine always has an oxidation number of −1. The other halogens have an oxidation number of −1 when they are negative; they can have positive oxidation numbers, Aqueous however, most notably in oxyanions. Reactions © 200 ...
- Angelo State University
... The Molar Mass of a Compound • The molar mass of a compound is obtained by adding together the atomic masses of all of the atoms in the molecule or formula unit. This number is either the mass of the compound in units of amu, or the mass of one mole of the compound in grams. – For molecular compound ...
... The Molar Mass of a Compound • The molar mass of a compound is obtained by adding together the atomic masses of all of the atoms in the molecule or formula unit. This number is either the mass of the compound in units of amu, or the mass of one mole of the compound in grams. – For molecular compound ...
Problem 1-2
... The top 15 of the 3rd round are the participants of the 4th round, a oneweek practical training. There are two written five-hour tests - one theoretical and one practical - under the same conditions as at the IChO. Here the team is selected. In this booklet all problems of the selection procedure an ...
... The top 15 of the 3rd round are the participants of the 4th round, a oneweek practical training. There are two written five-hour tests - one theoretical and one practical - under the same conditions as at the IChO. Here the team is selected. In this booklet all problems of the selection procedure an ...
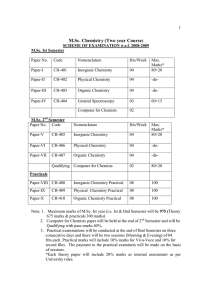
M.Sc. Chemistry (Two year Course)
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
- Kendriya Vidyalaya Jamuna Colliery
... 3. Niobium crystallizes in bcc structure. If its density is 8.55 cm-3, calculate atomic radius of [At. Mass of Niobium = 92.9u, NA = 6.022 x 1023 atoms mol-1 ]. 4. If radius of octahedral void is r and radius of atom in close packing is R, derive the relationship between r and R. 5. Non stoichiomet ...
... 3. Niobium crystallizes in bcc structure. If its density is 8.55 cm-3, calculate atomic radius of [At. Mass of Niobium = 92.9u, NA = 6.022 x 1023 atoms mol-1 ]. 4. If radius of octahedral void is r and radius of atom in close packing is R, derive the relationship between r and R. 5. Non stoichiomet ...
NICKEL(II) PINCER COMPLEXES SUPPORTED BY 2,6
... The starting material of the ligand synthesis, 2,6-dicarboxaldehye was prepared from 2,6-dimethanol pyridine through oxidation with SeO2/Dioxane (Figure 9). Then, tolualdehyde was stirred at room temperature for 5 h with 4-methylacetophenone and NaOH in EtOH/H2O to form 1,3-bis(4-tolyl)-2-propen-1-o ...
... The starting material of the ligand synthesis, 2,6-dicarboxaldehye was prepared from 2,6-dimethanol pyridine through oxidation with SeO2/Dioxane (Figure 9). Then, tolualdehyde was stirred at room temperature for 5 h with 4-methylacetophenone and NaOH in EtOH/H2O to form 1,3-bis(4-tolyl)-2-propen-1-o ...
Document
... the most interesting ions have a metal ion surrounded by a number of ligands. Ligands are molecules, such as ammonia, NH3, or anions, such as cyanide, CN −, that readily bond to metal ions. Figure 5 shows a model of one complex ion, [Cu(NH3)4]2+. Complex ions may be positively charged cations or neg ...
... the most interesting ions have a metal ion surrounded by a number of ligands. Ligands are molecules, such as ammonia, NH3, or anions, such as cyanide, CN −, that readily bond to metal ions. Figure 5 shows a model of one complex ion, [Cu(NH3)4]2+. Complex ions may be positively charged cations or neg ...
CB document - mvhs
... to chemistry. He discusses all three laws in detail and explains why they can be used to determine what is driving a reaction and if one will occur. This chapter clearly explains the major concepts of thermodynamics and is crucial to student understanding. The concluding chapter by Adele Mouakad has ...
... to chemistry. He discusses all three laws in detail and explains why they can be used to determine what is driving a reaction and if one will occur. This chapter clearly explains the major concepts of thermodynamics and is crucial to student understanding. The concluding chapter by Adele Mouakad has ...
Problem Authors - PianetaChimica
... The acids which are stronger than pure sulfuric acid are called superacids. Superacids are very strong proton donors being capable of protonating even weak Lewis acids such as Xe, H2, Cl 2, Br2, and CO2. Cations, which never exist in other media, have been observed in superacid solutions. George Ola ...
... The acids which are stronger than pure sulfuric acid are called superacids. Superacids are very strong proton donors being capable of protonating even weak Lewis acids such as Xe, H2, Cl 2, Br2, and CO2. Cations, which never exist in other media, have been observed in superacid solutions. George Ola ...
Writing Equilibrium Cons... and Liquids - Chemwiki
... H2O is one of the most common liquids dealt with in reactions. Remember to set its activity equal to 1 when it is a liquid in a reaction. However, if H2O is written as a gas, then its concentration must be considered. Knowing is very helpful, for when it is compared with the Reaction Quotient , whi ...
... H2O is one of the most common liquids dealt with in reactions. Remember to set its activity equal to 1 when it is a liquid in a reaction. However, if H2O is written as a gas, then its concentration must be considered. Knowing is very helpful, for when it is compared with the Reaction Quotient , whi ...
2 - C7Chemistry
... 2 moles of ammonia are produced, along with 1 mole of carbon dioxide and 1 mole of water vapor. ...
... 2 moles of ammonia are produced, along with 1 mole of carbon dioxide and 1 mole of water vapor. ...
105 ACID - DW Brooks
... was proposed by Br~lnsted and Lowry in 1923. This defmition, which gives a more complete picture of acids and bases, treated an acid as a proton donor and a base as a proton acceptor. An acid-base reaction then is essentially a transfer of protons. During the past several decades other useful defini ...
... was proposed by Br~lnsted and Lowry in 1923. This defmition, which gives a more complete picture of acids and bases, treated an acid as a proton donor and a base as a proton acceptor. An acid-base reaction then is essentially a transfer of protons. During the past several decades other useful defini ...
Syllabus Advanced Level and Advanced Subsidiary Level
... These are not listed in order of priority. Many of these Aims are reflected in the Assessment Objectives which follow; others are not readily assessed. The syllabus aims are to: ...
... These are not listed in order of priority. Many of these Aims are reflected in the Assessment Objectives which follow; others are not readily assessed. The syllabus aims are to: ...
Document
... • Ea is an energy barrier to the reaction • amount of energy needed to convert reactants into the activated complex aka transition state • the activated complex is a chemical species with partially broken and partially formed bonds always very high in energy because partial bonds ...
... • Ea is an energy barrier to the reaction • amount of energy needed to convert reactants into the activated complex aka transition state • the activated complex is a chemical species with partially broken and partially formed bonds always very high in energy because partial bonds ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.



2)](http://s1.studyres.com/store/data/015968611_1-56df287e8435abc2be6b0a2948d2417f-300x300.png)