SAMPLE PAPER -9 Time Allowed: 3 Hrs
... 9. For nitration , mixture of ConHNO3 , Con H2SO4 is used . When aniline is treated with Con H2SO4 it is oxidised to complex compounds due to high electron density on benzene ring. -NHCOCH3 decreases electron density on benzene ...
... 9. For nitration , mixture of ConHNO3 , Con H2SO4 is used . When aniline is treated with Con H2SO4 it is oxidised to complex compounds due to high electron density on benzene ring. -NHCOCH3 decreases electron density on benzene ...
e c n i
... Chemical reactions produce new substances that can usually be detected by observing the evidence: ...
... Chemical reactions produce new substances that can usually be detected by observing the evidence: ...
Spring 2014 Chemistry Review
... 16) Gaining three electrons gives what overall charge? 17) Draw the Lewis dot structure for N, K, & S2-. ...
... 16) Gaining three electrons gives what overall charge? 17) Draw the Lewis dot structure for N, K, & S2-. ...
File
... 25 CH3CH2COCH2CH3 reacts with hydrogen cyanide to form an organic product called a cyanohydrin. Which feature applies to the cyanohydrin product? A ...
... 25 CH3CH2COCH2CH3 reacts with hydrogen cyanide to form an organic product called a cyanohydrin. Which feature applies to the cyanohydrin product? A ...
Physical Science
... Chemical reactions take place when chemical bonds are either formed or broken. Strong chemical bonds resist change: glass Weak chemical bonds breakdown easily: wood ...
... Chemical reactions take place when chemical bonds are either formed or broken. Strong chemical bonds resist change: glass Weak chemical bonds breakdown easily: wood ...
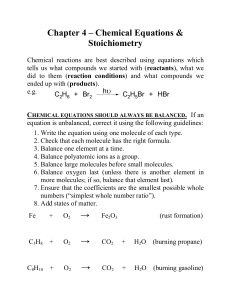
Reactions and Balancing
... coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent) – Think back to naming compounds/ determining formulas ...
... coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent) – Think back to naming compounds/ determining formulas ...
File
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
PS.Ch6.Test.95 - cloudfront.net
... 21. Consider the thermal energy transfer during a chemical process. When heat is transferred to the system, the process is said to be _______ and the sign of H is ________. a) exothermic, positive b) endothermic, negative c) exothermic, negative ...
... 21. Consider the thermal energy transfer during a chemical process. When heat is transferred to the system, the process is said to be _______ and the sign of H is ________. a) exothermic, positive b) endothermic, negative c) exothermic, negative ...
Honors Chemistry
... However, this is not the only driving force. Entropy (S): the measure of in a system. The higher disorder (more S), the likely the reaction is to occur (messy room, leaves on trees). Systems tend to go towards ...
... However, this is not the only driving force. Entropy (S): the measure of in a system. The higher disorder (more S), the likely the reaction is to occur (messy room, leaves on trees). Systems tend to go towards ...
Lecture 20 The Redox Sequence
... There is an ideal sequence of redox reactions driven by e- rich organic matter that is based on the energy available for the microbes that mediate the reactions. In this sequence organic matter is combusted in order by O2 → NO3 → MnO2 → Fe2O3 → SO42- (decreasing energy yield). Most of these reaction ...
... There is an ideal sequence of redox reactions driven by e- rich organic matter that is based on the energy available for the microbes that mediate the reactions. In this sequence organic matter is combusted in order by O2 → NO3 → MnO2 → Fe2O3 → SO42- (decreasing energy yield). Most of these reaction ...
3.10 Neutralization
... • Net ionic equations for reactions between strong acids and bases HCl(aq) + KOH(aq) → KCl(aq) + H2O(l) H+ + Cl- + K+ + OH- → K+ + Cl- + H2O(l) ⇒H+ + OH- → H2O(l) – H+ is present in the form of H3O+ ...
... • Net ionic equations for reactions between strong acids and bases HCl(aq) + KOH(aq) → KCl(aq) + H2O(l) H+ + Cl- + K+ + OH- → K+ + Cl- + H2O(l) ⇒H+ + OH- → H2O(l) – H+ is present in the form of H3O+ ...
Chapter 6
... oxidation-reduction reactions. In the metallurgy of galena (PbS), the principle lead-containing ore, the first step is the conversion of lead sulfide to its oxide (a process called roasting): 2PbS (s) + 3O2 (g) 2PbO (s) + 2SO2 (g) The oxide is then treated with carbon monoxide to produce the free ...
... oxidation-reduction reactions. In the metallurgy of galena (PbS), the principle lead-containing ore, the first step is the conversion of lead sulfide to its oxide (a process called roasting): 2PbS (s) + 3O2 (g) 2PbO (s) + 2SO2 (g) The oxide is then treated with carbon monoxide to produce the free ...
Click to download. - Life Learning Cloud
... made of only carbon, in hexagonal rings can be a football cage/tube structure used in medicine, where drugs put inside structure- used as delivery system to target specific parts of body and release drug also used as catalysts Giant Metallic Structures The atoms in metals are in layers which can ...
... made of only carbon, in hexagonal rings can be a football cage/tube structure used in medicine, where drugs put inside structure- used as delivery system to target specific parts of body and release drug also used as catalysts Giant Metallic Structures The atoms in metals are in layers which can ...
Storage Pattern for Chemicals Where Space is Limited
... Storage cabinets for acids, bases and flammables are meant for liquids, not dry solids. Vent acid cabinets to prevent vapor build-up. Store concentrated sulfuric acid on one shelf of the acid cabinet and concentrated hydrochloric acid on another. Store nitric acid in a secondary container with other ...
... Storage cabinets for acids, bases and flammables are meant for liquids, not dry solids. Vent acid cabinets to prevent vapor build-up. Store concentrated sulfuric acid on one shelf of the acid cabinet and concentrated hydrochloric acid on another. Store nitric acid in a secondary container with other ...
Rxn Types
... Single Displacement Reactions Not all single displacement reactions that can be written actually happen. The metal or non-metal must be more active than the ion it is replacing. It will depend upon the element’s Activity as ...
... Single Displacement Reactions Not all single displacement reactions that can be written actually happen. The metal or non-metal must be more active than the ion it is replacing. It will depend upon the element’s Activity as ...
Answers
... 5) A sample of ammonia (NH3) contains 7.22 moles of ammonia. How many molecules of ammonia are in the sample? 6) What is the mass of 2.0 mol of CuCl2? 7) A susbstance is analyzed and determined to be made up of 69.4 % carbon, 4.13 % hydrogen, and 26.4 % oxygen. The molar mass of the substance is fou ...
... 5) A sample of ammonia (NH3) contains 7.22 moles of ammonia. How many molecules of ammonia are in the sample? 6) What is the mass of 2.0 mol of CuCl2? 7) A susbstance is analyzed and determined to be made up of 69.4 % carbon, 4.13 % hydrogen, and 26.4 % oxygen. The molar mass of the substance is fou ...
Chapter 5 CHEM 121
... • The amounts of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. • In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers fro ...
... • The amounts of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. • In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers fro ...
Chemistry Standards Review
... (A) Temperature is determined by the average kinetic energy of particles (B) Molar heat capacity is related to the specific heat of a substance (C) Entropy is related to concentration (D) Temperature is determined by concentration 19. During the operation of a gasoline engine, the piston moves insid ...
... (A) Temperature is determined by the average kinetic energy of particles (B) Molar heat capacity is related to the specific heat of a substance (C) Entropy is related to concentration (D) Temperature is determined by concentration 19. During the operation of a gasoline engine, the piston moves insid ...
Paper
... levels of carbon dioxide emission per kilometre travelled will be subject to higher levels of taxation. The measures are designed to encourage the purchase of cars that are more fuel-efficient and have lower CO2 emissions. The manufacturer’s specification for a certain diesel-engined car is 143 g CO ...
... levels of carbon dioxide emission per kilometre travelled will be subject to higher levels of taxation. The measures are designed to encourage the purchase of cars that are more fuel-efficient and have lower CO2 emissions. The manufacturer’s specification for a certain diesel-engined car is 143 g CO ...
Chemistry 1A Final Exam December 12, 2001 Page 1 of 16 (Closed
... Equilibrium constants are smaller. Equilibrium constants are larger. Chemical reactions are sometimes more favorable. ...
... Equilibrium constants are smaller. Equilibrium constants are larger. Chemical reactions are sometimes more favorable. ...
Chapter 4 - U of L Class Index
... Quantitative analysis is the identification of an unknown substance by subjecting it to chemical reactions and analyzing the resulting products. (What are they? How much of each was made?) Generally, we must already know which elements the unknown contains in order to choose the best reactions. Quan ...
... Quantitative analysis is the identification of an unknown substance by subjecting it to chemical reactions and analyzing the resulting products. (What are they? How much of each was made?) Generally, we must already know which elements the unknown contains in order to choose the best reactions. Quan ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.