TYPES OF CHEMICAL REACTIONS AND SOLUTION CHEMISTRY
... 7. When the analyte is a base or an acid, the required titrant is a strong acid or strong base, respectively. This procedure is called an ______________________________. OXIDATION – REDUCTION REACTIONS 1. 2 Na (s) + Cl2 (g) → 2 NaCl (s) 2. Both reactants have no charge, they are neutral. NaCl is an ...
... 7. When the analyte is a base or an acid, the required titrant is a strong acid or strong base, respectively. This procedure is called an ______________________________. OXIDATION – REDUCTION REACTIONS 1. 2 Na (s) + Cl2 (g) → 2 NaCl (s) 2. Both reactants have no charge, they are neutral. NaCl is an ...
Semester II Exam Review Questions
... 3. Using the information from question (2) (the masses) and the balanced equation, calculate the theoretical amount of Tin (Sn) that should have been produced from this reaction. (hint: determine the limiting reactant) ...
... 3. Using the information from question (2) (the masses) and the balanced equation, calculate the theoretical amount of Tin (Sn) that should have been produced from this reaction. (hint: determine the limiting reactant) ...
Chemical Reactions
... The resultant barium sulfate must be disposed of properly. The two reactions above are straight forward acid/base neutralization reactions involving a strong acid and a strong base, but, many other very important reactions fall under the category of acid / base neutralization reactions. These includ ...
... The resultant barium sulfate must be disposed of properly. The two reactions above are straight forward acid/base neutralization reactions involving a strong acid and a strong base, but, many other very important reactions fall under the category of acid / base neutralization reactions. These includ ...
Instructions for AP/IB 2 Chem Summer Assignment Note
... covalent bonding and chemical reactions. This assignment will count as a major grade and is due the second day of class. It will be spot checked for correctness (i.e. it’s not just a completion grade; it will be scanned and points deducted for things that are obviously not even close to the correct ...
... covalent bonding and chemical reactions. This assignment will count as a major grade and is due the second day of class. It will be spot checked for correctness (i.e. it’s not just a completion grade; it will be scanned and points deducted for things that are obviously not even close to the correct ...
Chapter 2 Chemistry comes alive
... Atoms with six or seven valence shell electrons are electronegative Atoms with one or two valence shell electrons are electropositive ...
... Atoms with six or seven valence shell electrons are electronegative Atoms with one or two valence shell electrons are electropositive ...
Chapter 4_part 1
... Number the chain from the end that gives the lower numbers to the carbons of the C=C. Locate the C=C by the number of its first carbon. Use the ending -ene to show the presence of the C=C Branched-chain alkenes are named in a manner similar to alkanes in which substituted groups are located ...
... Number the chain from the end that gives the lower numbers to the carbons of the C=C. Locate the C=C by the number of its first carbon. Use the ending -ene to show the presence of the C=C Branched-chain alkenes are named in a manner similar to alkanes in which substituted groups are located ...
11-1 SECTION 11 THERMOCHEMISTRY Thermochemistry: Study of
... Most chemical reactions are accompanied by the release of energy to the surroundings or absorption of energy from the surroundings, or put more simply the reacting system gets hotter or cooler as the reaction proceeds. The most common form of energy transferred is heat. This section introduces the l ...
... Most chemical reactions are accompanied by the release of energy to the surroundings or absorption of energy from the surroundings, or put more simply the reacting system gets hotter or cooler as the reaction proceeds. The most common form of energy transferred is heat. This section introduces the l ...
01.CN_Other pages/p1-9
... extraction of metals from their ores. • Recognize that limestone, chalk and marble are different forms of calcium carbonate. • Study the weathering and erosion of rocks. • Explore the thermal decomposition of calcium carbonate. • Learn the tests for the presence of calcium and carbonate in a sample ...
... extraction of metals from their ores. • Recognize that limestone, chalk and marble are different forms of calcium carbonate. • Study the weathering and erosion of rocks. • Explore the thermal decomposition of calcium carbonate. • Learn the tests for the presence of calcium and carbonate in a sample ...
rate of chemical reaction and chemical equilibrium
... reaction solution becomes brownish. Concentration of iodine can be measured at different intervals of time by titration against ...
... reaction solution becomes brownish. Concentration of iodine can be measured at different intervals of time by titration against ...
Regents Chemistry Review Questions
... What is the chemical formula for ammonia? Is it an acid or a base? Write and balance the chemical equation for the neutralization reaction between carbonic acid and magnesium hydroxide. Name the salt that is produced in this reaction. Write and balance the chemical equation for the neutralization re ...
... What is the chemical formula for ammonia? Is it an acid or a base? Write and balance the chemical equation for the neutralization reaction between carbonic acid and magnesium hydroxide. Name the salt that is produced in this reaction. Write and balance the chemical equation for the neutralization re ...
Name ……………………………..………...… …….. Index No
... b) 20g of potassium chloride were placed in a glass beaker and 40.0cm3 of water were added. The beaker was heated until all the potassium chloride had dissolved and then allowed to cool. When crystals first appear the temperature was noted. An extra 5.0cm3 of water were added and the experiment was ...
... b) 20g of potassium chloride were placed in a glass beaker and 40.0cm3 of water were added. The beaker was heated until all the potassium chloride had dissolved and then allowed to cool. When crystals first appear the temperature was noted. An extra 5.0cm3 of water were added and the experiment was ...
No Slide Title
... In the previous single replacement reaction example, we have written the molecular equation for the reaction. Although this equation shows the reactants and products of the reaction, it does not give a very clear picture of what truly occurs in solution. In fact, such an aqueous solution actually co ...
... In the previous single replacement reaction example, we have written the molecular equation for the reaction. Although this equation shows the reactants and products of the reaction, it does not give a very clear picture of what truly occurs in solution. In fact, such an aqueous solution actually co ...
Energy Changes, Reaction Rates and Equilibrium Thermodynamics
... •The concentration of O2(g) will decrease. •The concentration of CO2(g) will increase. ...
... •The concentration of O2(g) will decrease. •The concentration of CO2(g) will increase. ...
Chapter 13…States of Matter
... 4. Calculate the amount of energy required to heat a 150 g chunk of aluminum from 20C to 40C. (Cp of aluminum = 0.220 cal/gC) H=mCpT (150g)(.22)(20) = 660 cal Chapters 17& 18…Reaction Rates & Equilibrium Define: 1. Equilibrium: the reaction occurs simultaneously in both directions. 2. Activate ...
... 4. Calculate the amount of energy required to heat a 150 g chunk of aluminum from 20C to 40C. (Cp of aluminum = 0.220 cal/gC) H=mCpT (150g)(.22)(20) = 660 cal Chapters 17& 18…Reaction Rates & Equilibrium Define: 1. Equilibrium: the reaction occurs simultaneously in both directions. 2. Activate ...
- Jersey College For Girls
... (ii) The reaction of sodium to form sodium oxide can be described as oxidation because it involves the addition of oxygen. State one other reason why this reaction can be described as oxidation. ...
... (ii) The reaction of sodium to form sodium oxide can be described as oxidation because it involves the addition of oxygen. State one other reason why this reaction can be described as oxidation. ...
Atomic Theory (2
... 1.) What are the 5 characteristics of ideal gases? 2.) What is the volume of one mole of any gas at STP? 3.) At what temperature would 2.10 moles of N2 gas have a pressure of 1.25 atm and in a 25.0 L tank? 4.) What volume is occupied by 5.03 g of O2 at 28°C and a pressure of 0.998atm? 5.) What is th ...
... 1.) What are the 5 characteristics of ideal gases? 2.) What is the volume of one mole of any gas at STP? 3.) At what temperature would 2.10 moles of N2 gas have a pressure of 1.25 atm and in a 25.0 L tank? 4.) What volume is occupied by 5.03 g of O2 at 28°C and a pressure of 0.998atm? 5.) What is th ...
Part I - American Chemical Society
... (A) Ag3PO4 (Ksp = 1 × 10-16) (B) Ca3(PO4)2 (Ksp = 1 × 10-33) (C) Mg3(PO4)2 (Ksp = 1 × 10-24) (D) AlPO4 (Ksp = 1 × 10-20) 36. Which salt is significantly more soluble in a strong acid than in water? ...
... (A) Ag3PO4 (Ksp = 1 × 10-16) (B) Ca3(PO4)2 (Ksp = 1 × 10-33) (C) Mg3(PO4)2 (Ksp = 1 × 10-24) (D) AlPO4 (Ksp = 1 × 10-20) 36. Which salt is significantly more soluble in a strong acid than in water? ...
1st Olympiad of Metropolises Chemistry Theoretical Problems
... under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory, the sequence of furan hydrolysis followed by Paal-Knorr reaction with ammonia (amine) is used for this transformation. This sequence can be realized as a two-step procedure or as a domino reaction. ...
... under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory, the sequence of furan hydrolysis followed by Paal-Knorr reaction with ammonia (amine) is used for this transformation. This sequence can be realized as a two-step procedure or as a domino reaction. ...
IB Definitions
... Nanotechnology involves research and technology development at the 1 nm - to - 100 nm range. Nanotechnology creates and uses structures that have novel properties because of their small size. Nanotechnology builds on the ability to control or manipulate at the atomic scale.” Food chemistry Define th ...
... Nanotechnology involves research and technology development at the 1 nm - to - 100 nm range. Nanotechnology creates and uses structures that have novel properties because of their small size. Nanotechnology builds on the ability to control or manipulate at the atomic scale.” Food chemistry Define th ...
Chemistry- CST Review
... 1. What causes gas pressure in terms of kinetic theory? Gas pressure is caused by the random motion of the gas molecules. 2. If someone sprays perfume at the front of the room, will the people in the back of the room eventually be able to smell it? Why? Explain completely. Yes, the perfume will be s ...
... 1. What causes gas pressure in terms of kinetic theory? Gas pressure is caused by the random motion of the gas molecules. 2. If someone sprays perfume at the front of the room, will the people in the back of the room eventually be able to smell it? Why? Explain completely. Yes, the perfume will be s ...
Chapters 18 – The Periodic Table
... ns2p4 electron configuration (n = 2 to 6) Metallic properties increase down the column, but no G6A element behaves like a true metal Common behavior: reaction with metal to become 2- ion in ionic compound; for most metals, most common minerals are oxides or sulfides Covalent bonds with other ...
... ns2p4 electron configuration (n = 2 to 6) Metallic properties increase down the column, but no G6A element behaves like a true metal Common behavior: reaction with metal to become 2- ion in ionic compound; for most metals, most common minerals are oxides or sulfides Covalent bonds with other ...
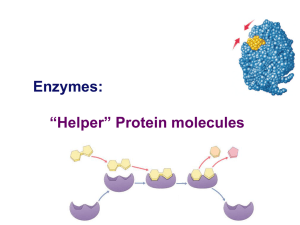
Enzymes: “Helper” Protein molecules
... Enzymes aren’t used up Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
... Enzymes aren’t used up Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
Topic2890 Thermodynamics and Kinetics A given system at
... reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, dξ/dt, it is not immediately obvious ∂A how one might estimate the affinity A and ...
... reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, dξ/dt, it is not immediately obvious ∂A how one might estimate the affinity A and ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.