CHEM1405 2012-J-2 June 2012 • What is the ground state electron
... Pyridine is the most basic as the lone pair of electrons on nitrogen is available to bond with H+. Pyrrole is the least basic as the “lone pair” of electrons on nitrogen is part of the aromatic π-electron system and is delocalised around the ring. It is not available for bonding with H+ ions. N-Phen ...
... Pyridine is the most basic as the lone pair of electrons on nitrogen is available to bond with H+. Pyrrole is the least basic as the “lone pair” of electrons on nitrogen is part of the aromatic π-electron system and is delocalised around the ring. It is not available for bonding with H+ ions. N-Phen ...
*6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing
... *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that alters the form or appearance of a substance but does not change it into another ...
... *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that alters the form or appearance of a substance but does not change it into another ...
Equilibrium
... l. If the cylinders A and B represented the reactants and products in a chemical reaction, which of the cylinders represented the reactants and which would represent the products? Explain how you came to this conclusion. Saying you guessed is not an acceptable answer. Model 2: Disturbing Equilibrium ...
... l. If the cylinders A and B represented the reactants and products in a chemical reaction, which of the cylinders represented the reactants and which would represent the products? Explain how you came to this conclusion. Saying you guessed is not an acceptable answer. Model 2: Disturbing Equilibrium ...
Unit 14.1 REDOX Reactions Objectives REDOX Reactions
... • REDOX reactions involve the transfer of electrons from one species to another. • A REDOX reaction involves both an oxidation of one species and a reduction of another. • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
... • REDOX reactions involve the transfer of electrons from one species to another. • A REDOX reaction involves both an oxidation of one species and a reduction of another. • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
Review Session 3 Problems
... 1) Titanium tetrachloride is an important industrial chemical. It is used for preparing the TiO2, the white pigment in paints and paper. It can be made using an impure titanium ore(often impure TiO2) with carbon and chlorine. ...
... 1) Titanium tetrachloride is an important industrial chemical. It is used for preparing the TiO2, the white pigment in paints and paper. It can be made using an impure titanium ore(often impure TiO2) with carbon and chlorine. ...
Types of reactions: redox reactions
... • H2 + F2 → 2HF can be re-written as two half-reactions: H2 → 2H + + 2e− (oxidation) and F2 + 2e− → 2F − (reduction) • Cl2 +2KI → 2KCl+I2 or Cl2 +2I − → 2Cl− +I2 , can be written as two half-reactions: Cl2 +2e− → 2Cl− ...
... • H2 + F2 → 2HF can be re-written as two half-reactions: H2 → 2H + + 2e− (oxidation) and F2 + 2e− → 2F − (reduction) • Cl2 +2KI → 2KCl+I2 or Cl2 +2I − → 2Cl− +I2 , can be written as two half-reactions: Cl2 +2e− → 2Cl− ...
chemical reaction
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
Synthesis of Aliphatic Nitro Compounds1i2 A simple new
... Preparation of %nitrooctane from b-iodo~ctane.~2-Iodooctane (71.2 g., 0.30 mole) was poured into a stirred solution of 225 ml. dimethyl sulfoxide (DMSO) and 36 g. of sodium nitrite (0.52 mole) contained in a 500 ml. flask immersed in a water bath held a t room temperature. Stirring was continued for ...
... Preparation of %nitrooctane from b-iodo~ctane.~2-Iodooctane (71.2 g., 0.30 mole) was poured into a stirred solution of 225 ml. dimethyl sulfoxide (DMSO) and 36 g. of sodium nitrite (0.52 mole) contained in a 500 ml. flask immersed in a water bath held a t room temperature. Stirring was continued for ...
Chemical reactions and equations
... reactants and products 3) Add any symbols needed for state of matter, catalysts, heat, etc. 4) Count the number of atoms of each element. Include ...
... reactants and products 3) Add any symbols needed for state of matter, catalysts, heat, etc. 4) Count the number of atoms of each element. Include ...
C2_revision_slides_V3_+_questions_+_MS_-_H[1]
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
Ionic bonding
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
Ionic bonding - Animated Science
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
C2 revision slides V3 + questions + MS
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
... Carbon can also form fullerenes with different numbers of carbon atoms. They are used for drug delivery into the body, lubricants, catalysts, and in nanotubes for reinforcing materials, eg tennis rackets. ...
Chemistry 520 - Problem Set 6
... helix-coil transition? Both of these observations can be explained by the interactions of the two forms of the polypeptide with the solvent. If more solvent molecules are bound to the coiled form than the helix then the reaction will be exothermic (products are at lower internal energy than the reac ...
... helix-coil transition? Both of these observations can be explained by the interactions of the two forms of the polypeptide with the solvent. If more solvent molecules are bound to the coiled form than the helix then the reaction will be exothermic (products are at lower internal energy than the reac ...
General Chemistry Sample Exam 2 and Outline
... ii) What is the limiting reagent and how much remains if 35 ml of 6.0 M sulfuric acid is spilled and 50 grams of sodium bicarbonate is added ? iii) What is the mass of carbon dioxide gas (g) that is produced ? iv) How many molecules of carbon dioxide are produced ? v) If 5.00 ml of water is actually ...
... ii) What is the limiting reagent and how much remains if 35 ml of 6.0 M sulfuric acid is spilled and 50 grams of sodium bicarbonate is added ? iii) What is the mass of carbon dioxide gas (g) that is produced ? iv) How many molecules of carbon dioxide are produced ? v) If 5.00 ml of water is actually ...
Ch 3 Chemical Reactions 2013-Sept-08
... Metal Sulfides are black and metal sulfides come from the center of the earth. Sulfides are insoluble in water so they form a black mass in the deep ocean floor cracks. Chemical Reactions are the heart of Chemistry. This chapter is an introduction to symbols and chemical reactions. 3.1 Intro to Chem ...
... Metal Sulfides are black and metal sulfides come from the center of the earth. Sulfides are insoluble in water so they form a black mass in the deep ocean floor cracks. Chemical Reactions are the heart of Chemistry. This chapter is an introduction to symbols and chemical reactions. 3.1 Intro to Chem ...
Chemistry - StudyTime NZ
... Neither Oxygen nor Magnesium have full valence electron shells. Because of this, they must each lose or gain electrons in order to become stable. Oxygen has 8 electrons and hence an electron arrangement ...
... Neither Oxygen nor Magnesium have full valence electron shells. Because of this, they must each lose or gain electrons in order to become stable. Oxygen has 8 electrons and hence an electron arrangement ...
Welcome to AP Chemistry!
... 1. The oxidation number of any uncombined element is O. 2. The oxidation number of a monatomic ion equal the charge on the ion. 3. The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in ...
... 1. The oxidation number of any uncombined element is O. 2. The oxidation number of a monatomic ion equal the charge on the ion. 3. The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in ...
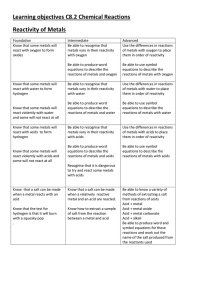
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... Use the differences in reactions of metals with oxygen to place them in order of reactivity ...
... Use the differences in reactions of metals with oxygen to place them in order of reactivity ...
Unit 13, Lesson 1
... A metal in a compound can be displaced by another metal in the elemental state. We have already seen example of copper replacing silver ions in our famous demonstration. If we reversed the roles of the metals—added pure silver to copper nitrate solution, no reaction would happen. How do we know this ...
... A metal in a compound can be displaced by another metal in the elemental state. We have already seen example of copper replacing silver ions in our famous demonstration. If we reversed the roles of the metals—added pure silver to copper nitrate solution, no reaction would happen. How do we know this ...
Document
... Reactant 1 + Reactant 2 Product 1 + Product 2 (the number of reactants and products will vary) ...
... Reactant 1 + Reactant 2 Product 1 + Product 2 (the number of reactants and products will vary) ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.










![C2_revision_slides_V3_+_questions_+_MS_-_H[1]](http://s1.studyres.com/store/data/000092833_1-97fb33725e7f1ef12029ed42751d3dca-300x300.png)