Exam No. 1
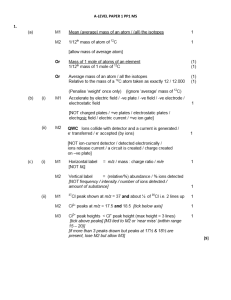
... sample of nitrogen at 25oC and 160 torr are both placed in a 200 mL flask at 25oC. What is the final pressure in the 200 mL flask? (a) (c) ...
... sample of nitrogen at 25oC and 160 torr are both placed in a 200 mL flask at 25oC. What is the final pressure in the 200 mL flask? (a) (c) ...
1 - A-Level Chemistry
... Mass of zinc lost = 0.05 × 65.4 = 3.27 g Correct final answer without working scores M3 only. ...
... Mass of zinc lost = 0.05 × 65.4 = 3.27 g Correct final answer without working scores M3 only. ...
turcuman s - Revista de Chimie
... CoL2 x 2H2O compounds, it results that in both cases the values of a and c parameters are relatively close though they are much lower than the b value. This shows that the central atoms of Fe(III) and Co(II) coordinates with H2O respectively in the ac plan of the elemental cell towards b side accomp ...
... CoL2 x 2H2O compounds, it results that in both cases the values of a and c parameters are relatively close though they are much lower than the b value. This shows that the central atoms of Fe(III) and Co(II) coordinates with H2O respectively in the ac plan of the elemental cell towards b side accomp ...
Kitchen Chemistry Review
... Be able to recognize if a change is physical or chemical, giving reasons why. Physical: Change in shape, size, state or amount Chemical: Change in chemical composition, new substance is made. ...
... Be able to recognize if a change is physical or chemical, giving reasons why. Physical: Change in shape, size, state or amount Chemical: Change in chemical composition, new substance is made. ...
pH scale. Buffer solutions. Colligative properties of solutions
... Buffer solutions The amount of carbon dioxide in the blood is coupled to the amount present in the lungs. Second, the equilibrium between carbonic acid and bicarbonate ion: H2CO3 (aq) ↔ HCO3− (aq) + H+ (aq), pK = 6.37. These reactions lead to the presence in solution the conjugate pair HCO3−/H2CO3 ...
... Buffer solutions The amount of carbon dioxide in the blood is coupled to the amount present in the lungs. Second, the equilibrium between carbonic acid and bicarbonate ion: H2CO3 (aq) ↔ HCO3− (aq) + H+ (aq), pK = 6.37. These reactions lead to the presence in solution the conjugate pair HCO3−/H2CO3 ...
Advanced Higher Chemistry Resource Guide
... Those attending felt it would be useful to have a document which helped them navigate to the most relevant resources quickly. The following pages show the mandatory course key areas table from the SQA Advanced Higher Chemistry Course and Unit Support Notes. An additional fourth column has been inclu ...
... Those attending felt it would be useful to have a document which helped them navigate to the most relevant resources quickly. The following pages show the mandatory course key areas table from the SQA Advanced Higher Chemistry Course and Unit Support Notes. An additional fourth column has been inclu ...
Journal of Molecular Catalysis A: Chemical Enhancing
... Monohalogenation of diols [34] is often a stubborn problem in different synthetic endeavoures, as there is considerable formation of disubstituted product. Immiscibility of ionic liquid with different organic solvents, and their tendency to form biphasic media, could offer an excellent solution for ...
... Monohalogenation of diols [34] is often a stubborn problem in different synthetic endeavoures, as there is considerable formation of disubstituted product. Immiscibility of ionic liquid with different organic solvents, and their tendency to form biphasic media, could offer an excellent solution for ...
U3 Student Workbook - The Connected Chemistry Curriculum
... of Mass to chemical equations by learning how to balance them. Following a teacher demonstration of the simulation and procedures, students will use the simulations to look at ten different reactions. In each of the reactions, students will create submicroscopic sketches and balance the chemical for ...
... of Mass to chemical equations by learning how to balance them. Following a teacher demonstration of the simulation and procedures, students will use the simulations to look at ten different reactions. In each of the reactions, students will create submicroscopic sketches and balance the chemical for ...
Acids, Bases, and pH
... pH. Log indicates how many times 10 is multiplied by itself to result in the number [H+]. This means that for every increase in 1 pH unit, there is a 10x increase in [H+]. The logarithmic relationship between [H+] and pH is shown in Fig 6. ...
... pH. Log indicates how many times 10 is multiplied by itself to result in the number [H+]. This means that for every increase in 1 pH unit, there is a 10x increase in [H+]. The logarithmic relationship between [H+] and pH is shown in Fig 6. ...
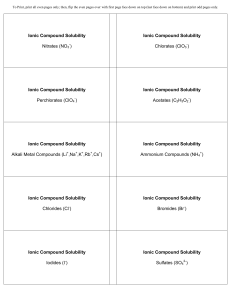
Ionic Compound Solubility Nitrates (NO3 ) Ionic Compound
... Oxygen and a metallic element. Ex: Fe2O3 Oxygen and a nonmentallic element. ...
... Oxygen and a metallic element. Ex: Fe2O3 Oxygen and a nonmentallic element. ...
Chemistry Summer Work (30 questions):
... of alkali metals, alkali earth metals, halogens, noble gases, transition metal,: element sodium: ...
... of alkali metals, alkali earth metals, halogens, noble gases, transition metal,: element sodium: ...
print
... The reverse reaction is negligible at a point soon after the reactants are mixed. Therefore the reaction rate will only depend on the concentration of the reactants. Rate = k[NO2]n k = rate constant n = rate order (an integer or a fraction) ...
... The reverse reaction is negligible at a point soon after the reactants are mixed. Therefore the reaction rate will only depend on the concentration of the reactants. Rate = k[NO2]n k = rate constant n = rate order (an integer or a fraction) ...
2.ATOMS, MOLECULES, AND IONS
... chamber where the experimenter follows the motion of one drop with a microscope. Some of these drops have picked up one or more electrons as a result of friction in the atomizer and have become negatively charged. A negatively charged drop will be attracted upwards when the experimenter turns on a c ...
... chamber where the experimenter follows the motion of one drop with a microscope. Some of these drops have picked up one or more electrons as a result of friction in the atomizer and have become negatively charged. A negatively charged drop will be attracted upwards when the experimenter turns on a c ...
bond enthalpy activity sheet
... How many litres of nitrogen dioxide gas would be produced in a reaction, starting with a mixture of 5 litres of nitrogen monoxide gas and 2 litres of oxygen gas? (All volumes are measures under the same conditions of temperature and pressure.) ...
... How many litres of nitrogen dioxide gas would be produced in a reaction, starting with a mixture of 5 litres of nitrogen monoxide gas and 2 litres of oxygen gas? (All volumes are measures under the same conditions of temperature and pressure.) ...
Higher Chemistry - Mobile Resource
... have a ‘hard’ collision in which bonds will break and allow the reaction to happen. Clearly there must be a minimum speed (or KE) of collision at which the reaction can just happen. This minimum necessary Kinetic Energy is called the Activation Energy. If the temperature of a reaction is raised the ...
... have a ‘hard’ collision in which bonds will break and allow the reaction to happen. Clearly there must be a minimum speed (or KE) of collision at which the reaction can just happen. This minimum necessary Kinetic Energy is called the Activation Energy. If the temperature of a reaction is raised the ...
Equilibrium STUDY GUIDE by Keshara Senanayake ---
... If both sides have equal # of moles of gas a shift in either direction will not reduce the pressure. So a increase in pressure has no effect on the equilibrium situation. So an equilibrium reaction that has the same number of moles of gas on both sides of the equation will not be affected by the cha ...
... If both sides have equal # of moles of gas a shift in either direction will not reduce the pressure. So a increase in pressure has no effect on the equilibrium situation. So an equilibrium reaction that has the same number of moles of gas on both sides of the equation will not be affected by the cha ...
Chapter 7
... chemical reaction. • It may change form (solid to liquid or gas). • If 1 atom of Carbon goes into a reaction, 1 atom of carbon must come out. It can’t be lost or multiplied. ...
... chemical reaction. • It may change form (solid to liquid or gas). • If 1 atom of Carbon goes into a reaction, 1 atom of carbon must come out. It can’t be lost or multiplied. ...
mark scheme - A-Level Chemistry
... Do not allow ‘to improve accuracy’ without qualification. Do not allow ‘water is a product of the reaction’. Do not allow ‘mass of crucible incorrect / too high’. ...
... Do not allow ‘to improve accuracy’ without qualification. Do not allow ‘water is a product of the reaction’. Do not allow ‘mass of crucible incorrect / too high’. ...
Discrete Electromagnetics: Maxwell`s Equations Tailored to
... differential formulation of Maxwell’s equations. With the introduction of computers, new mathematical tools and engineering disciplines had to be devised to actually solve the electromagnetics equations. Since digital computer arithmetics typically just allows to process a finite number of real and ...
... differential formulation of Maxwell’s equations. With the introduction of computers, new mathematical tools and engineering disciplines had to be devised to actually solve the electromagnetics equations. Since digital computer arithmetics typically just allows to process a finite number of real and ...
08 Redox Reactions
... no useful electrical work could be obtained. In these reactions, chemical energy appears as heat. If the transferance of electrons from zinc to copper ions is allowed to occur through some metallic wires, useful electrical work could be performed. Such redox reactions are called Indirect redox react ...
... no useful electrical work could be obtained. In these reactions, chemical energy appears as heat. If the transferance of electrons from zinc to copper ions is allowed to occur through some metallic wires, useful electrical work could be performed. Such redox reactions are called Indirect redox react ...
Critical Review Microbial Electrolysis Cells for High Yield Hydrogen
... costs associated with the large surface areas that are required. It was independently discovered by two different research groups a few years ago that bacteria could be used to make hydrogen gas in an electrolysis-type process based on microbial fuel cells (MFCs) (5–7). In an MFC, bacteria oxidize o ...
... costs associated with the large surface areas that are required. It was independently discovered by two different research groups a few years ago that bacteria could be used to make hydrogen gas in an electrolysis-type process based on microbial fuel cells (MFCs) (5–7). In an MFC, bacteria oxidize o ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.