OXIDATION NUMBERS
... A combination of two ionic half equations, one involving oxidation and the other reduction, produces a balanced REDOX equation. The equations can be balanced as follows... ...
... A combination of two ionic half equations, one involving oxidation and the other reduction, produces a balanced REDOX equation. The equations can be balanced as follows... ...
Chemistry 199 - Oregon State chemistry
... sides of the reaction arrow is 60 and the atomic number on both sides of the reaction arrow is 27. This corresponds to 6027Co. 60m27Co is in the excited state and will emit a gamma ray to become 6027Co. A student isolates a sample of tritium containing 1,000 atoms. What will be the number of tritium ...
... sides of the reaction arrow is 60 and the atomic number on both sides of the reaction arrow is 27. This corresponds to 6027Co. 60m27Co is in the excited state and will emit a gamma ray to become 6027Co. A student isolates a sample of tritium containing 1,000 atoms. What will be the number of tritium ...
chemistry
... oxidation states (2) colorless ions in solution, multiple negative oxidation states (3) colored ions in solution, multiple positive oxidation states (4) colored ions in solution, multiple negative oxidation states ...
... oxidation states (2) colorless ions in solution, multiple negative oxidation states (3) colored ions in solution, multiple positive oxidation states (4) colored ions in solution, multiple negative oxidation states ...
Early Atomic Models
... caused deflection of the beam. This was eventually accomplished by J.J. Thomson. The rays were believed to be streams of particles. Thomson named them electrons and changed the model of the atom. ...
... caused deflection of the beam. This was eventually accomplished by J.J. Thomson. The rays were believed to be streams of particles. Thomson named them electrons and changed the model of the atom. ...
Unit IV: Nature of Matter
... caused deflection of the beam. This was eventually accomplished by J.J. Thomson. The rays were believed to be streams of particles. Thomson named them electrons and changed the model of the atom. ...
... caused deflection of the beam. This was eventually accomplished by J.J. Thomson. The rays were believed to be streams of particles. Thomson named them electrons and changed the model of the atom. ...
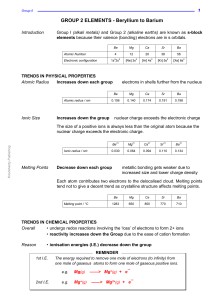
GROUP 2 ELEMENTS - Beryllium to Barium
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
Chemistry Unit Review
... b. Lead (II) iodide and potassium nitrate are produced when potassium iodide is added to lead (II) nitrate. ...
... b. Lead (II) iodide and potassium nitrate are produced when potassium iodide is added to lead (II) nitrate. ...
3.10 Neutralization
... ZnS(s) + 2HCl(aq) → ZnCl2(aq) + H2S(g) ZnS(s) + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2S(g) ⇒ZnS(s) + 2H+ → Zn2+ + H2S(g) – H+ is present in the form of H3O+ ...
... ZnS(s) + 2HCl(aq) → ZnCl2(aq) + H2S(g) ZnS(s) + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2S(g) ⇒ZnS(s) + 2H+ → Zn2+ + H2S(g) – H+ is present in the form of H3O+ ...
double-replacement reaction
... • These elements are written as diatomic molecules when they appear in chemical reactions. ...
... • These elements are written as diatomic molecules when they appear in chemical reactions. ...
Eighth Grade Review - PAMS-Doyle
... ions. When acids dissolve in water, hydrogen ions (H+) are released into the resulting solution. A base is a substance that releases hydroxide ions (OH–) into solution. ...
... ions. When acids dissolve in water, hydrogen ions (H+) are released into the resulting solution. A base is a substance that releases hydroxide ions (OH–) into solution. ...
Electromagnetic Fields and their effect on Biological systems
... cell's cytoskeleton are green. During prometaphase the chromosomes (which contain the DNA) align along the microtubule spindle (green lines across centre). The spindles drag the chromosomes apart, forming two identical sets. Mitosis is the process by which a cell divides into two identical daughter ...
... cell's cytoskeleton are green. During prometaphase the chromosomes (which contain the DNA) align along the microtubule spindle (green lines across centre). The spindles drag the chromosomes apart, forming two identical sets. Mitosis is the process by which a cell divides into two identical daughter ...
(Tungsten)–Nickel (Cobalt) Alloys and Intermetallic Compounds
... lattices and the difference in their standard electrode potentials are of great importance. It seems significant from a practical point of view to study the electrodeposition of alloys whose components have crystalline lattices of different types but similar electrode potentials. The analysis of the ...
... lattices and the difference in their standard electrode potentials are of great importance. It seems significant from a practical point of view to study the electrodeposition of alloys whose components have crystalline lattices of different types but similar electrode potentials. The analysis of the ...
Naming Ionic Compounds
... this is harder because you must balance out the electronic charges to zero! use these rules: o write down the symbols for the elements in the order they appear in the compound name – the metal will always be first and the non metal second e.g. calcium chloride…. write down CaCl o figure out the ioni ...
... this is harder because you must balance out the electronic charges to zero! use these rules: o write down the symbols for the elements in the order they appear in the compound name – the metal will always be first and the non metal second e.g. calcium chloride…. write down CaCl o figure out the ioni ...
Chemical Equations & Reactions
... Active metals displace less active metals or hydrogen from their compounds in aqueous solution. Refer to the Activity Series. a. Al + CuCl2 AlCl3 + Cu b. metal + H2O metal hydroxide + H2 Metals include the alkali metals and calcium. 2Na + 2H2O 2NaOH + H2 ...
... Active metals displace less active metals or hydrogen from their compounds in aqueous solution. Refer to the Activity Series. a. Al + CuCl2 AlCl3 + Cu b. metal + H2O metal hydroxide + H2 Metals include the alkali metals and calcium. 2Na + 2H2O 2NaOH + H2 ...
activity series
... 3. All binary compounds of the halogens (other than F) with metals are soluble, except those of Ag, Hg (I), and Pb. (Pb halides are soluble in hot water.) ...
... 3. All binary compounds of the halogens (other than F) with metals are soluble, except those of Ag, Hg (I), and Pb. (Pb halides are soluble in hot water.) ...
Precipitate Lab Report Power Point with Answers
... one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no obvious “evidence” from TOPIC-B. Precipitates occur when a double replacement reaction happens. To start, you need 2 aqueous solutions. Aqueous means that the compound is dissolve ...
... one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no obvious “evidence” from TOPIC-B. Precipitates occur when a double replacement reaction happens. To start, you need 2 aqueous solutions. Aqueous means that the compound is dissolve ...
+ H 2 SO 4(aq) - Rothschild Science
... What do the equations mean? The coefficients indicate the number of moles of the reactants necessary… to form a certain mole of the product. Mole to Mole Ratio ...
... What do the equations mean? The coefficients indicate the number of moles of the reactants necessary… to form a certain mole of the product. Mole to Mole Ratio ...
Answers
... ____ a) they must be equal ____ b) the mass of the products must be greater ____ c) the mass of the reactants must be greater ____ d) there is no general relationship between the two 15) In balancing a chemical equation, which of the following are you allowed to do? ____ a) change subscripts ____ b) ...
... ____ a) they must be equal ____ b) the mass of the products must be greater ____ c) the mass of the reactants must be greater ____ d) there is no general relationship between the two 15) In balancing a chemical equation, which of the following are you allowed to do? ____ a) change subscripts ____ b) ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.