Inorganic Chemistry
... uses and limitations. Debye-Hückel theory, equation for strong electrolytes (elementary treatment only). Migration of ions, Transport number, definition and determination by Hittorf and moving boundary methods, Kohlrausch’s law. Application of conductivity measurementsdetermination of degree of diss ...
... uses and limitations. Debye-Hückel theory, equation for strong electrolytes (elementary treatment only). Migration of ions, Transport number, definition and determination by Hittorf and moving boundary methods, Kohlrausch’s law. Application of conductivity measurementsdetermination of degree of diss ...
materials: metals and non—metals
... hydrogen. The reaction is slow at room temperature, but its rate can be increased by the addition of a little copper (II) sulphate. Zinc displaces copper metal, which acts as a catalyst. In general, ...
... hydrogen. The reaction is slow at room temperature, but its rate can be increased by the addition of a little copper (II) sulphate. Zinc displaces copper metal, which acts as a catalyst. In general, ...
Units 3 and 4 Revision
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
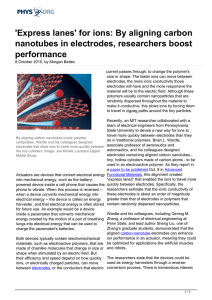
`Express lanes` for ions: By aligning carbon nanotubes in
... Zhang, a professor of electrical engineering at Penn State, and lead author Sheng Liu, one of Zhang's graduate students, demonstrated that the aligned carbon-nanotube electrodes can enhance ion performance in an actuator, meaning they could Both devices typically contain electromechanical materials, ...
... Zhang, a professor of electrical engineering at Penn State, and lead author Sheng Liu, one of Zhang's graduate students, demonstrated that the aligned carbon-nanotube electrodes can enhance ion performance in an actuator, meaning they could Both devices typically contain electromechanical materials, ...
Ionic Compounds 1. What is the formula for aluminum phosphate
... Solutions – Like Dissolves Like, Molarity, Reaction Types, Solubility 1. An unknown substance dissolves in water but not in benzene (a nonpolar solvent). Molecules of what type are present in the substance? 2. A 87.2-g sample of SrCl2 is dissolved in 112.5 mL of solution. Calculate the molarity of ...
... Solutions – Like Dissolves Like, Molarity, Reaction Types, Solubility 1. An unknown substance dissolves in water but not in benzene (a nonpolar solvent). Molecules of what type are present in the substance? 2. A 87.2-g sample of SrCl2 is dissolved in 112.5 mL of solution. Calculate the molarity of ...
Worksheet for 4-29 - Iowa State University
... 6. Cathodic protection: add a ____ (more/less) reactive metal such as ___ or ___ that preferentially oxidizes and forces the iron to be the ______ (anode/cathode) and therefore remains in its reduced form. Such metals must have a more _______ (positive/negative) Eored potential. ...
... 6. Cathodic protection: add a ____ (more/less) reactive metal such as ___ or ___ that preferentially oxidizes and forces the iron to be the ______ (anode/cathode) and therefore remains in its reduced form. Such metals must have a more _______ (positive/negative) Eored potential. ...
Lecture 03B - Balancing Redox
... • Reduction is the gain of electrons by a chemical process. • When Cl- ions are formed from elemental chlorine, chlorine is reduced. ...
... • Reduction is the gain of electrons by a chemical process. • When Cl- ions are formed from elemental chlorine, chlorine is reduced. ...
Chapter 7 Chemical Reactions
... 4. pure copper and sulfur dioxide are produced by heating copper( II) sulfide in the presence of oxygen 5, Water is formed by the explosive reaction between hydrogen and oxygen 7.2 Writing Chemical Equations In chemistry to communicate more effectively chemical formulas are used to write chemical ...
... 4. pure copper and sulfur dioxide are produced by heating copper( II) sulfide in the presence of oxygen 5, Water is formed by the explosive reaction between hydrogen and oxygen 7.2 Writing Chemical Equations In chemistry to communicate more effectively chemical formulas are used to write chemical ...
Chemistry- CST Review
... 4. Calculate the molarity of each of the following solutions: a) 0.60 mol of NaCl dissolved in 1.6 L of solution. b) 25.2 g of potassium nitrate, KNO3, in enough water to make 150.0 mL of solution. 5. Calculate the number of grams of solute needed to prepare each of the following solutions: a) 4500. ...
... 4. Calculate the molarity of each of the following solutions: a) 0.60 mol of NaCl dissolved in 1.6 L of solution. b) 25.2 g of potassium nitrate, KNO3, in enough water to make 150.0 mL of solution. 5. Calculate the number of grams of solute needed to prepare each of the following solutions: a) 4500. ...
EKSIKA JOINT EVALUATION TEST. Kenya Certificate
... Given that the lattice enthalpy of potassium chloride is +690KJ/mol and hydration enthalpies of K+ and Cl- are -322KJ and -364KJ respectively. Calculate the enthalpy of solution of potassium chloride. ...
... Given that the lattice enthalpy of potassium chloride is +690KJ/mol and hydration enthalpies of K+ and Cl- are -322KJ and -364KJ respectively. Calculate the enthalpy of solution of potassium chloride. ...
Pre-AP Chemistry - Simple Rules for Electron Exchange Simple
... Metals lose electrons when they become ions. Nonmetals gain electrons when they become ions. Second Rule Number of electrons gained by the reduced species must equal number of electrons lost by the oxidized species. Third Rule Polyatomic ions that are not broken up during chemical reactions are usua ...
... Metals lose electrons when they become ions. Nonmetals gain electrons when they become ions. Second Rule Number of electrons gained by the reduced species must equal number of electrons lost by the oxidized species. Third Rule Polyatomic ions that are not broken up during chemical reactions are usua ...
Chapter 11 Chemical Reactions
... 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
... 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
REACTION DYNAMICS
... is slightly endothermic for v=0. The following data were obtained for the reaction cross section σr at ...
... is slightly endothermic for v=0. The following data were obtained for the reaction cross section σr at ...
Chemical Reactions
... Two things replace each other. – Reactants must be two ionic compounds, in aqueous solution ...
... Two things replace each other. – Reactants must be two ionic compounds, in aqueous solution ...
13.2 Chemical Formulas
... atoms of hydrogen and one atom of oxygen to build the molecule. For sodium nitrate, NaNO3, the chemical formula tells us there are three elements in the compound: sodium (Na), nitrogen (N), and oxygen (O). To make a molecule of this compound, you need one atom of sodium, one atom of nitrogen, and th ...
... atoms of hydrogen and one atom of oxygen to build the molecule. For sodium nitrate, NaNO3, the chemical formula tells us there are three elements in the compound: sodium (Na), nitrogen (N), and oxygen (O). To make a molecule of this compound, you need one atom of sodium, one atom of nitrogen, and th ...
In-Class Exam - Fayetteville State University
... 16. There are _______ atoms of oxygen in 300 molecules of CH3CO2H. A) 1.80x1026 B) 300 C) 3.01x1024 D) 600 E) 6.02x1023 17. A compound that is composed of only carbon and hydrogen contains 85.7% C and 14.3% H by mass. What is the empirical formula of the compound? A) CH2 B) C2H4 C) C4H8 D) CH4 18. A ...
... 16. There are _______ atoms of oxygen in 300 molecules of CH3CO2H. A) 1.80x1026 B) 300 C) 3.01x1024 D) 600 E) 6.02x1023 17. A compound that is composed of only carbon and hydrogen contains 85.7% C and 14.3% H by mass. What is the empirical formula of the compound? A) CH2 B) C2H4 C) C4H8 D) CH4 18. A ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.