2007 - SolPass
... written permission from the copyright owner. Commonwealth of Virginia public school educators may reproduce any portion of these released tests for non-commercial educational purposes without requesting permission. All others should direct their written requests to the Virginia Department of Educati ...
... written permission from the copyright owner. Commonwealth of Virginia public school educators may reproduce any portion of these released tests for non-commercial educational purposes without requesting permission. All others should direct their written requests to the Virginia Department of Educati ...
Chapter 10 The Periodic Law
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the eleme ...
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the eleme ...
Title - Iowa State University
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
Chemistry 2202 Background Information – Chapter 1 (pg
... Matter and the Atom – Pg 12-21 The Atom – The smallest particle of an element that still maintains the properties of that element. (fig. 1.7, pg 12) Atomic mass unit (u) – unit of mass that is 1/12 of the mass of a unit of carbon-12 atom; equal to 1.66x10-24 g. Atomic number (Z) – refers to the numb ...
... Matter and the Atom – Pg 12-21 The Atom – The smallest particle of an element that still maintains the properties of that element. (fig. 1.7, pg 12) Atomic mass unit (u) – unit of mass that is 1/12 of the mass of a unit of carbon-12 atom; equal to 1.66x10-24 g. Atomic number (Z) – refers to the numb ...
Lecture 10 Activity of chemical components
... where Z is the charge on the ion and I is called as net ionic strength, ci is the concentration of the i th ion. The factor –0.0509 depends on the solvent dielectric constant and temperature. Thus we can see that Debye model predicts a reduction in activity coefficients. (1) Higher the charge, lower ...
... where Z is the charge on the ion and I is called as net ionic strength, ci is the concentration of the i th ion. The factor –0.0509 depends on the solvent dielectric constant and temperature. Thus we can see that Debye model predicts a reduction in activity coefficients. (1) Higher the charge, lower ...
Chemical Reactions
... Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
Chemical Reactions
... The activation energy is the energy needed to start the reaction. When particles collide with sufficient energy – at least equal to the activation energy – existing bonds may be disrupted and new bonds can form Endothermic reaction – the energy of the product is greater than that of the reactants (e ...
... The activation energy is the energy needed to start the reaction. When particles collide with sufficient energy – at least equal to the activation energy – existing bonds may be disrupted and new bonds can form Endothermic reaction – the energy of the product is greater than that of the reactants (e ...
Science 10 Chem - Holy Trinity Academy
... Element: a substance that cannot be broken down any further by a chemical reaction into any simpler substance. pure substances that contain a single kind of atom Each element differs from the others because it has distinct physical and chemical properties ...
... Element: a substance that cannot be broken down any further by a chemical reaction into any simpler substance. pure substances that contain a single kind of atom Each element differs from the others because it has distinct physical and chemical properties ...
Electricity
... • Parallel: a circuit in which the parts are joined in branches such that the potential difference across each part is the same • Unlike series, each load does not have the same current ...
... • Parallel: a circuit in which the parts are joined in branches such that the potential difference across each part is the same • Unlike series, each load does not have the same current ...
section_2_review_set
... 1. What is the claim to fame for the proton? 2. What is the claim to fame for the electron? 3. What is the claim to fame for the neutron? 4. What is the mass of each of the following particles?: proton; neutron; electron. 5. What is the charge for each of the following particles?: proton; neutron; e ...
... 1. What is the claim to fame for the proton? 2. What is the claim to fame for the electron? 3. What is the claim to fame for the neutron? 4. What is the mass of each of the following particles?: proton; neutron; electron. 5. What is the charge for each of the following particles?: proton; neutron; e ...
Aqueous Reactions and Solution Stoichiometry (Chapter 4)
... Water has many unique chemical and physical properties. Possibly one of the most important is its ability to dissolve other substances to form solutions. Solutions are homogeneous mixtures of two or more substances. The solvent (usually the substance present in the greatest quantity) causes the othe ...
... Water has many unique chemical and physical properties. Possibly one of the most important is its ability to dissolve other substances to form solutions. Solutions are homogeneous mixtures of two or more substances. The solvent (usually the substance present in the greatest quantity) causes the othe ...
Topic2890 Thermodynamics and Kinetics A given system at
... reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, dξ/dt, it is not immediately obvious ∂A how one might estimate the affinity A and ...
... reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, dξ/dt, it is not immediately obvious ∂A how one might estimate the affinity A and ...
Pauling Scale of Electronegativities for the Various Elements
... OA OA RA OA or RA Remember, oxygen at oxidation number -2 almost never acts as a reducing agent; therefore, it can be ignored throughout the remainder of this problem. Because the potassium (K+2) and manganese (MN+7) can only behave as oxidizing agents, C11 must be the reducing agent. Step 3: ...
... OA OA RA OA or RA Remember, oxygen at oxidation number -2 almost never acts as a reducing agent; therefore, it can be ignored throughout the remainder of this problem. Because the potassium (K+2) and manganese (MN+7) can only behave as oxidizing agents, C11 must be the reducing agent. Step 3: ...
Advanced Placement Chemistry: 1984 Free Response Questions
... (d) The addition of antifreeze to water in a radiator decreases the likelihood that the liquid in the radiator will either freeze or boil. ...
... (d) The addition of antifreeze to water in a radiator decreases the likelihood that the liquid in the radiator will either freeze or boil. ...
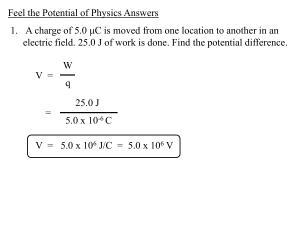
Feel the Potential of Physics Answers
... Feel the Potential of Physics Answers 1. A charge of 5.0 μC is moved from one location to another in an electric field. 25.0 J of work is done. Find the potential difference. ...
... Feel the Potential of Physics Answers 1. A charge of 5.0 μC is moved from one location to another in an electric field. 25.0 J of work is done. Find the potential difference. ...
CHM1 Exam 16 Name 2222222222222222222222222222 Multiple
... (1) 19 protons and 23 neutrons (2) 19 protons and 42 neutrons (3) 20 protons and 19 neutrons (4) 23 protons and 19 neutrons ...
... (1) 19 protons and 23 neutrons (2) 19 protons and 42 neutrons (3) 20 protons and 19 neutrons (4) 23 protons and 19 neutrons ...
Stoichiometry - WordPress.com
... • Solutions are measured by their volume and the concentration of substance dissolved in them, known as molarity (M). This has the unit mol/L. • A solution of concentration 2.5 M means it has 2.5 mol of the solute dissolved in 1 litre of solvent. • The formula used for solution stoichiometry is: ...
... • Solutions are measured by their volume and the concentration of substance dissolved in them, known as molarity (M). This has the unit mol/L. • A solution of concentration 2.5 M means it has 2.5 mol of the solute dissolved in 1 litre of solvent. • The formula used for solution stoichiometry is: ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.