Free Energy I
... Burning two moles of H2 in O2 (under conditions of constant P) would decrease the entropy of the system by 326.3 J/K for every mole of O2 or every two moles of water or H2. How much does the entropy change when 0.275 g of H2 is ignited with O2 in a constant-pressure system? ...
... Burning two moles of H2 in O2 (under conditions of constant P) would decrease the entropy of the system by 326.3 J/K for every mole of O2 or every two moles of water or H2. How much does the entropy change when 0.275 g of H2 is ignited with O2 in a constant-pressure system? ...
chapter28.3 - Colorado Mesa University
... A negative charge is moving through an electric field along a path consisting of 2 legs (A & B). Let W represent the work done by the field, and ΔV the change in potential. Which of the following statements is/are true: ...
... A negative charge is moving through an electric field along a path consisting of 2 legs (A & B). Let W represent the work done by the field, and ΔV the change in potential. Which of the following statements is/are true: ...
Chapter 7
... Energy in Chemical Reactions Heat and other natural processes in a system always tend toward less usable energy and greater disorder… This is known as the second law of thermodynamics When you eat something, only about 55% of energy is actually used…the rest is converted into heat and “lost” to you ...
... Energy in Chemical Reactions Heat and other natural processes in a system always tend toward less usable energy and greater disorder… This is known as the second law of thermodynamics When you eat something, only about 55% of energy is actually used…the rest is converted into heat and “lost” to you ...
Chemistry Module 1- Basic Revision Notes 1.1a Atomic Structure 1.1
... reactivity down the group, they also, are known as soft metals(i.e. can be cut easily by a knife) are low in density(i.e. they even float on water) are stored under paraffin (oil) due to their high reactivity with water react quickly with oxygen (in the air)and tarnish are shiny when newly ...
... reactivity down the group, they also, are known as soft metals(i.e. can be cut easily by a knife) are low in density(i.e. they even float on water) are stored under paraffin (oil) due to their high reactivity with water react quickly with oxygen (in the air)and tarnish are shiny when newly ...
Lecture 11 - U of L Class Index
... For example, CaO melts at 2572°C, a temperature well beyond the range of an ordinary fire. Calcium compounds such as lime (CaO) were known and used in ancient times. Calcium metal, however, was first prepared in 1808 by Sir Humphry Davy, who also prepared magnesium, strontium, and barium in the same ...
... For example, CaO melts at 2572°C, a temperature well beyond the range of an ordinary fire. Calcium compounds such as lime (CaO) were known and used in ancient times. Calcium metal, however, was first prepared in 1808 by Sir Humphry Davy, who also prepared magnesium, strontium, and barium in the same ...
11U CHEMISTRY EXAM REVIEW QUESTIONS June 2010
... 3. A compound contains 56.6% potassium, 8.7% carbon and 34.7% oxygen. Determine the empirical formula for the compound. 4. Hydroquinone is an organic compound commonly used as a photographic developer. It has a molecular mass of 110 g/mol and a composition of C3H3O. Calculate the molecular formula o ...
... 3. A compound contains 56.6% potassium, 8.7% carbon and 34.7% oxygen. Determine the empirical formula for the compound. 4. Hydroquinone is an organic compound commonly used as a photographic developer. It has a molecular mass of 110 g/mol and a composition of C3H3O. Calculate the molecular formula o ...
Document
... The standard reduction potentials for Ce (IV) and CO2 are: Ce4+ + e Ce3+ E = 1.72 2 CO2 + 2H+ + 2e H2C2O4 E = -0.481 ...
... The standard reduction potentials for Ce (IV) and CO2 are: Ce4+ + e Ce3+ E = 1.72 2 CO2 + 2H+ + 2e H2C2O4 E = -0.481 ...
Chapter 10 Handouts_1
... The electrons in an atom that have the same principal quantum number n occupy the same shell. The electrons in an atom that have the same orbital quantum number l occupy the same subshell. The larger the value of l, the more electrons the subshell can hold. A shell or subshell that contains its full ...
... The electrons in an atom that have the same principal quantum number n occupy the same shell. The electrons in an atom that have the same orbital quantum number l occupy the same subshell. The larger the value of l, the more electrons the subshell can hold. A shell or subshell that contains its full ...
Chapter 10_Handouts_6
... remain together during chemical reactions. The sulfate group SO4 is an example of an atom group. A precipitate is an insoluble solid that results from a chemical reaction in solution. When two or more atom groups of the same kind are present in the formula of a compound, parentheses are placed aroun ...
... remain together during chemical reactions. The sulfate group SO4 is an example of an atom group. A precipitate is an insoluble solid that results from a chemical reaction in solution. When two or more atom groups of the same kind are present in the formula of a compound, parentheses are placed aroun ...
Exam 3 Review
... 1. draw the Lewis dot structure 2. draw circles around each atom and the electrons associated with it. Remember that formal charges are associated with covalent bonds and that all electrons are shared equally. 3. compare to the group number for that atom. If the number is larger the formal charge is ...
... 1. draw the Lewis dot structure 2. draw circles around each atom and the electrons associated with it. Remember that formal charges are associated with covalent bonds and that all electrons are shared equally. 3. compare to the group number for that atom. If the number is larger the formal charge is ...
No Slide Title
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
$doc.title
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
FINAL EXAM REVIEW
... 4. With respect to electrons, how does an ionic bond differ from a covalent bond? 5. Indicate whether the following compounds are ionic, nonpolar covalent, or polar covalent. Explain. a) NaCl b) H2O c) NO2 d) CS2 6. How many valence electrons are there in: a) Si b) K+1 c) Ne ...
... 4. With respect to electrons, how does an ionic bond differ from a covalent bond? 5. Indicate whether the following compounds are ionic, nonpolar covalent, or polar covalent. Explain. a) NaCl b) H2O c) NO2 d) CS2 6. How many valence electrons are there in: a) Si b) K+1 c) Ne ...
Describing Chemical Reactions
... Chemical reaction: the process by which one or more substances are changed into one or more new substances Represented by chemical equations Chemical equation: a shorthand expression that represents a chemical reaction Shows the relative amount of each substance taking place in a chemical re ...
... Chemical reaction: the process by which one or more substances are changed into one or more new substances Represented by chemical equations Chemical equation: a shorthand expression that represents a chemical reaction Shows the relative amount of each substance taking place in a chemical re ...
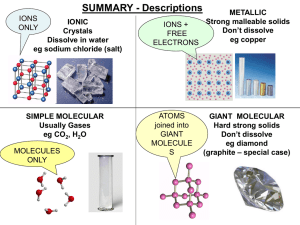
smart_materials_1 - Aldercar High School
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
Chapter 5 CHEM 121
... • The amounts of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. • In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers fro ...
... • The amounts of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. • In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers fro ...
Column A
... a. Calcium and Chlorine- CaCl2 b. Magnesium and oxygen- MgO c. Magnesium and phosphorus- Mg3P2 G) Write the formulas for the following compounds. ...
... a. Calcium and Chlorine- CaCl2 b. Magnesium and oxygen- MgO c. Magnesium and phosphorus- Mg3P2 G) Write the formulas for the following compounds. ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.