Ch17-2 Driving Forces of Reactions
... A chemical reaction in which the products Can react to reform the the reactants. When is a reaction that is reversible considered to have reached chemical equilibrium? When rate of forward reaction = rate of reverse reaction Equilibrium constant: Keq A numerical ratio of the concentration of the pro ...
... A chemical reaction in which the products Can react to reform the the reactants. When is a reaction that is reversible considered to have reached chemical equilibrium? When rate of forward reaction = rate of reverse reaction Equilibrium constant: Keq A numerical ratio of the concentration of the pro ...
In this chapter, alkanes, alkenes, alkynes
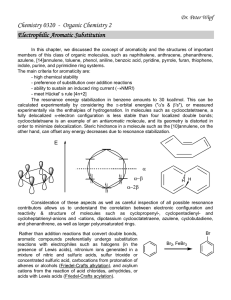
... azulene, [14]annulene, toluene, phenol, aniline, benzoic acid, pyridine, pyrrole, furan, thiophene, indole, purine, and pyrimidine ring systems. The main criteria for aromaticity are: - high chemical stability - preference of substitution over addition reactions - ability to sustain an induced ring ...
... azulene, [14]annulene, toluene, phenol, aniline, benzoic acid, pyridine, pyrrole, furan, thiophene, indole, purine, and pyrimidine ring systems. The main criteria for aromaticity are: - high chemical stability - preference of substitution over addition reactions - ability to sustain an induced ring ...
CHM 2045C - State College of Florida
... This course meets Area V for the A.A./A.S. general education requirements. A rigorous study of chemistry principles for students who have already studied basic concepts of chemistry. This course is intended for science and science-related majors. ...
... This course meets Area V for the A.A./A.S. general education requirements. A rigorous study of chemistry principles for students who have already studied basic concepts of chemistry. This course is intended for science and science-related majors. ...
ppt
... specific heat of solution of KBrO3 (s) is an endothermic 0.25 kJ/g. Calculate the molar heat of solution and write the ...
... specific heat of solution of KBrO3 (s) is an endothermic 0.25 kJ/g. Calculate the molar heat of solution and write the ...
8th Grade Ch. 7 Chemical Reactions Study guide
... 6. Activation energy is the minimum amount of energy needed for a chemical reaction to begin. ...
... 6. Activation energy is the minimum amount of energy needed for a chemical reaction to begin. ...
Atomic Emissions LAB Questions
... THEY SHOW SPECTRAL LINES OF COLOR REPRESENTING ELECTRONS MOVING FROM THE EXCITED STATE TO THE GROUND STATE. CONTRAST— EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have ...
... THEY SHOW SPECTRAL LINES OF COLOR REPRESENTING ELECTRONS MOVING FROM THE EXCITED STATE TO THE GROUND STATE. CONTRAST— EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have ...
Text S1.
... by using the Gaussian simulation package 032. Molecular electrostatic potential for each conformer was calculated by using density functional theory (DFT) method B3LYP with the ccpVTZ basis set. The IEFPCM continuum solvent model was employed to imitate an organic solvent environment (= 4). Atomic ...
... by using the Gaussian simulation package 032. Molecular electrostatic potential for each conformer was calculated by using density functional theory (DFT) method B3LYP with the ccpVTZ basis set. The IEFPCM continuum solvent model was employed to imitate an organic solvent environment (= 4). Atomic ...
Chemical Reactions
... process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as the law of conservation of mass ...
... process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as the law of conservation of mass ...
Vocabulary Terms Defined
... “electromagnetic spectrum” of an object has a different meaning, and is instead the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object. wavelength (91) () is the distance between corresponding points on adjacent waves. frequency (92) (f; The textb ...
... “electromagnetic spectrum” of an object has a different meaning, and is instead the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object. wavelength (91) () is the distance between corresponding points on adjacent waves. frequency (92) (f; The textb ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... standard integral 0 exp(-bx2) dx = (1/2)(/b)1/2.] 19. Define or explain the three parts that make an atomic term symbol and formulate the term symbols for the ground state configuration of F atom. 20. In solving the H2+ problem using the LCAO method, the lowest energy obtained is given by E+ = (H ...
... standard integral 0 exp(-bx2) dx = (1/2)(/b)1/2.] 19. Define or explain the three parts that make an atomic term symbol and formulate the term symbols for the ground state configuration of F atom. 20. In solving the H2+ problem using the LCAO method, the lowest energy obtained is given by E+ = (H ...
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
... The synthesis of new molecules that can work as chiral ligands in metal catalyzed enantioselective reactions is currently an area of great interest.1,2 These ligands must be capable of coordinating with the metal ion, generating around it a highly asymmetric environment so that the complex formed is ...
... The synthesis of new molecules that can work as chiral ligands in metal catalyzed enantioselective reactions is currently an area of great interest.1,2 These ligands must be capable of coordinating with the metal ion, generating around it a highly asymmetric environment so that the complex formed is ...
Week - Mat-Su School District
... iii. Oxidation-reduction (redox) 1. Oxidation numbers 2. The electrons role 3. Electrochemistry, electrolytic & galvanic cells, Faradays laws, standard half-cell potentials, Nernst equation ...
... iii. Oxidation-reduction (redox) 1. Oxidation numbers 2. The electrons role 3. Electrochemistry, electrolytic & galvanic cells, Faradays laws, standard half-cell potentials, Nernst equation ...
Handout - EnvLit - Michigan State University
... How do K-12 students from the US and China reason about carbon-transforming processes? How do American and Chinese students progress with respect to reasoning about carbon-transforming processes from elementary to high school? ...
... How do K-12 students from the US and China reason about carbon-transforming processes? How do American and Chinese students progress with respect to reasoning about carbon-transforming processes from elementary to high school? ...
Chemistry I Final Review
... 12. How many electrons, protons and neutrons in a: a. sodium-23 ion ...
... 12. How many electrons, protons and neutrons in a: a. sodium-23 ion ...
Chapter 1
... C. The Importance of Accuracy D. The Reason Equations Must be Balanced *Notes-The Law of Conservation of Mass dictates that chemical equations must be balanced because atoms are never _____lost__or _____gained__in a chemical reaction. ...
... C. The Importance of Accuracy D. The Reason Equations Must be Balanced *Notes-The Law of Conservation of Mass dictates that chemical equations must be balanced because atoms are never _____lost__or _____gained__in a chemical reaction. ...
Notes on Heat, temperature and kinetic energy
... • …is released when chemical bonds are broken and stored when bonds are formed • …comes in many forms including light, heat and electricity, chemical, sound ...
... • …is released when chemical bonds are broken and stored when bonds are formed • …comes in many forms including light, heat and electricity, chemical, sound ...