Atoms in Combination: The Chemical Bond
... Calcium and chlorine neutral-atom electron configurations (left), and their configurations after electrons have been transferred from the calcium to the chlorine atoms (right). ...
... Calcium and chlorine neutral-atom electron configurations (left), and their configurations after electrons have been transferred from the calcium to the chlorine atoms (right). ...
Curriculum Plan
... equations, Define standard conditions for standard enthalpy change and its notation, State Hess’s law, Use Hess’s law to find Ho for a reaction, Describe the process of calorimetry, Define rate of reaction, Identify intermediate products of a reaction, Describe a rate law for a chemical reaction, D ...
... equations, Define standard conditions for standard enthalpy change and its notation, State Hess’s law, Use Hess’s law to find Ho for a reaction, Describe the process of calorimetry, Define rate of reaction, Identify intermediate products of a reaction, Describe a rate law for a chemical reaction, D ...
Document
... The standard free-energy of reaction (DG0rxn) is the freeenergy change for a reaction when it occurs under standardstate conditions. ...
... The standard free-energy of reaction (DG0rxn) is the freeenergy change for a reaction when it occurs under standardstate conditions. ...
Writing and Balancing Chemical Equations
... only C, H, (and maybe O) is reacted with oxygen – usually called “burning” If the combustion is complete, the products will be CO2 and H2O. If the combustion is incomplete, the products will be CO (or possibly just C) and H2O. ...
... only C, H, (and maybe O) is reacted with oxygen – usually called “burning” If the combustion is complete, the products will be CO2 and H2O. If the combustion is incomplete, the products will be CO (or possibly just C) and H2O. ...
Solution-Phase Combinatorial Chemistry
... isolation and purification. • It was first used for easily synthesized compound classes [amides, sulfonamides, ureas, heterocycles (thiazole)]. • Presently, solution-phase combinatorial synthesis is attracting more interest because of some advantages. ...
... isolation and purification. • It was first used for easily synthesized compound classes [amides, sulfonamides, ureas, heterocycles (thiazole)]. • Presently, solution-phase combinatorial synthesis is attracting more interest because of some advantages. ...
Standard answers: 1 Basic concepts, Fuels, alkanes and alkenes
... Provides alternative route with a lower Ea More particles have E > new lower Ea More successful collisions ...
... Provides alternative route with a lower Ea More particles have E > new lower Ea More successful collisions ...
Document
... **Flip the first equation, as the product of that reaction is the reactant in our problem reaction. At the same time, the sign in front of the ΔH needs to be reversed as well. P4O6 P4 + 3O2 ...
... **Flip the first equation, as the product of that reaction is the reactant in our problem reaction. At the same time, the sign in front of the ΔH needs to be reversed as well. P4O6 P4 + 3O2 ...
Ab initio studies on optimized geometries for the thiazole
... that charge transfer occurs within the molecule. The geometries and energies obtained from HF/6-31G* calculations are in good agreement with the experimentally observed data. Keywords: N,N’-bis(2-Thiazol-yl)methylenediamin, HF, HOMO, LOMO. ____________________________________________________________ ...
... that charge transfer occurs within the molecule. The geometries and energies obtained from HF/6-31G* calculations are in good agreement with the experimentally observed data. Keywords: N,N’-bis(2-Thiazol-yl)methylenediamin, HF, HOMO, LOMO. ____________________________________________________________ ...
Exam 2-f06 - Clayton State University
... 8.) The equilibrium constant, Kc for the following gas phase reaction is 0.50 at 600°C. A mixture of HCHO, H and CO is introduced into a flask at 600°C. After a short time, analysis of a small amount of the reaction mixture shows the concentration to be [HCHO] = 1.5M, [H2] = 1.2 M and [CO] = 1.0M. W ...
... 8.) The equilibrium constant, Kc for the following gas phase reaction is 0.50 at 600°C. A mixture of HCHO, H and CO is introduced into a flask at 600°C. After a short time, analysis of a small amount of the reaction mixture shows the concentration to be [HCHO] = 1.5M, [H2] = 1.2 M and [CO] = 1.0M. W ...
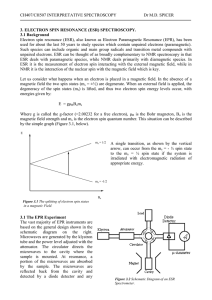
ESR Theory - Personal WWW Pages
... as 10-9 M can be detected in favourable cases. This is in contrast to NMR which ideally requires samples of at least 10-2 M. ESR is also versatile, in that a range of sample types can be studied. It is best for magnetically dilute systems, where the paramagnetic species is surrounded by diamagnetic ...
... as 10-9 M can be detected in favourable cases. This is in contrast to NMR which ideally requires samples of at least 10-2 M. ESR is also versatile, in that a range of sample types can be studied. It is best for magnetically dilute systems, where the paramagnetic species is surrounded by diamagnetic ...
Title
... Atomic and molecular structure - wave mechanical model of the atom, description of atomic orbitals, molecular orbitals and bonding, solid state chemistry, packing, radius ratio, crystal lattices. Physical Chemistry (normally 11 hours) Introduction to formal chemical kinetics: reaction rates and calc ...
... Atomic and molecular structure - wave mechanical model of the atom, description of atomic orbitals, molecular orbitals and bonding, solid state chemistry, packing, radius ratio, crystal lattices. Physical Chemistry (normally 11 hours) Introduction to formal chemical kinetics: reaction rates and calc ...
2 - DrChoChemistryWebSite
... We can’t remember them all, but luckily they will fall into several categories. We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
... We can’t remember them all, but luckily they will fall into several categories. We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
Chemistry I Review - BarbaraElam-Rice
... 30) An atom has 2 electrons in its valence shell. To what group does the atom belong? Will the atom for an anion or a cation? What will be the oxidation number of the ion? 31) An intermolecular force that holds ionic compounds together is called electrostatic attraction. 32) Describe the 3 intermole ...
... 30) An atom has 2 electrons in its valence shell. To what group does the atom belong? Will the atom for an anion or a cation? What will be the oxidation number of the ion? 31) An intermolecular force that holds ionic compounds together is called electrostatic attraction. 32) Describe the 3 intermole ...
File
... 1. Write separate equations for oxidation and reduction half reactions 2. For each half-reaction A. balance all the elements B. balance oxygen using H2O C. balance hydrogen using H+ D. balance the charge using electrons 3. if necessary, multiply one or both half-reactions by an integer to equalize t ...
... 1. Write separate equations for oxidation and reduction half reactions 2. For each half-reaction A. balance all the elements B. balance oxygen using H2O C. balance hydrogen using H+ D. balance the charge using electrons 3. if necessary, multiply one or both half-reactions by an integer to equalize t ...
File
... Essential Idea: Physical and chemical properties depend on the way in which different atoms combine ...
... Essential Idea: Physical and chemical properties depend on the way in which different atoms combine ...