CHEMISTRY 1000 - U of L Class Index
... a second, more permanent green solid, Cu2(OH)2SO4 is produced: Cu2(OH)2CO3(s) + H2SO4(aq) Cu2(OH)2SO4(s) + H2CO3(aq) ...
... a second, more permanent green solid, Cu2(OH)2SO4 is produced: Cu2(OH)2CO3(s) + H2SO4(aq) Cu2(OH)2SO4(s) + H2CO3(aq) ...
Chapter 8powerp point for chemical reactions
... List observations that suggest that a chemical reaction has taken place. List three requirements for a correctly written chemical equation. Write a word equation and a formula equation for a given chemical reaction. Balance a formula equation by inspection. ...
... List observations that suggest that a chemical reaction has taken place. List three requirements for a correctly written chemical equation. Write a word equation and a formula equation for a given chemical reaction. Balance a formula equation by inspection. ...
CAPE CHEMISTRY UNIT TWO REVISION PAPER MODULE 1 (a
... matches the energy difference between the lower energy state (ground state) and one of the higher energy state of the atoms or molecules. Ultra-violet and visible spectroscopy deals with the absorption of energy bringing about an increase in the energy of electrons. These energy changes are quantize ...
... matches the energy difference between the lower energy state (ground state) and one of the higher energy state of the atoms or molecules. Ultra-violet and visible spectroscopy deals with the absorption of energy bringing about an increase in the energy of electrons. These energy changes are quantize ...
Preface - Wiley Online Library
... a ring in service of uniting separate carbon atoms in that ring to form alkenes. These so called “extrusion reactions” are more commonly known for the loss of a single molecular fragment as in, e.g. the Ramberg-B¨acklund Reaction which has been reviewed twice in this series (L. Paquette, Volume 25 a ...
... a ring in service of uniting separate carbon atoms in that ring to form alkenes. These so called “extrusion reactions” are more commonly known for the loss of a single molecular fragment as in, e.g. the Ramberg-B¨acklund Reaction which has been reviewed twice in this series (L. Paquette, Volume 25 a ...
Honors-Final-Review-2014
... _____ boiling point _____ surfactant _____ viscosity _____ solution _____ surface tension ...
... _____ boiling point _____ surfactant _____ viscosity _____ solution _____ surface tension ...
Chemistry Of The Human Body
... • Structure of atoms. • Atomic number Vs atomic mass. • Role of electron shells ...
... • Structure of atoms. • Atomic number Vs atomic mass. • Role of electron shells ...
Chemistry Of The Human Body
... • Structure of atoms. • Atomic number Vs atomic mass. • Role of electron shells ...
... • Structure of atoms. • Atomic number Vs atomic mass. • Role of electron shells ...
CHEMISTRY 1.2 LECTURE
... II. Predicting E° (potential) and the direction of a Redox Reaction A redox reaction is the sum of two half-reactions, one for oxidation and the other for reduction Consider the following: ...
... II. Predicting E° (potential) and the direction of a Redox Reaction A redox reaction is the sum of two half-reactions, one for oxidation and the other for reduction Consider the following: ...
KIN1PP - Knockhardy
... One can look at the QUALITATIVE and the QUANTITATIVE aspects of how the rate (speed) of a reaction can be changed. Chemical kinetics plays an important part in industrial chemistry because the time taken for a reaction to take place and the energy required are of great economic importance. The kinet ...
... One can look at the QUALITATIVE and the QUANTITATIVE aspects of how the rate (speed) of a reaction can be changed. Chemical kinetics plays an important part in industrial chemistry because the time taken for a reaction to take place and the energy required are of great economic importance. The kinet ...
3. atomic structure
... in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to ide ...
... in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to ide ...
Energy of Reactions
... There is potential and kinetic energy Energy can neither be created or destroyed Every compound needs energy to increase temperature or to change from one state of matter to another ...
... There is potential and kinetic energy Energy can neither be created or destroyed Every compound needs energy to increase temperature or to change from one state of matter to another ...
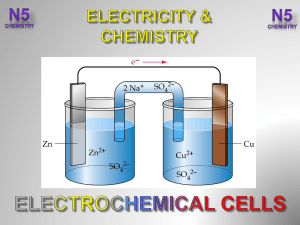
3.-Electrochemical-Cells-V2-
... are batteries which, when they go ‘flat’, can be charged and re-used. This means during recharging the chemicals used in the redox reactions are reformed. The lead-acid battery is the oldest type of rechargeable battery. The battery is made from plates and ...
... are batteries which, when they go ‘flat’, can be charged and re-used. This means during recharging the chemicals used in the redox reactions are reformed. The lead-acid battery is the oldest type of rechargeable battery. The battery is made from plates and ...
Bio_130_files/Chemistry Review
... • All types of matter (solids, liquids and gases) are composed of atoms. • A substance that is composed of only one type of atom is called an element. – Elements are the simplest form of matter with unique chemical properties. They are charted on the periodic table based on some of their chemical ch ...
... • All types of matter (solids, liquids and gases) are composed of atoms. • A substance that is composed of only one type of atom is called an element. – Elements are the simplest form of matter with unique chemical properties. They are charted on the periodic table based on some of their chemical ch ...