COMPOUNDS Chapter 2 : Preliminary course Chemistry 2
... of outer shell electrons and hence a positive valency ...
... of outer shell electrons and hence a positive valency ...
Chemistry Chapter 4 Vocabulary 1. Solution 2. Solute 3. Solvent 4
... the concentration of hydrogen ions in a solution measurement of acidity describing a solution that is neither an acid nor a base substance that has a pH of 7 A metal made of 2 or more solids 2 or more substances mixed together but not chemically combined Homogenous – particles/ substances are evenly ...
... the concentration of hydrogen ions in a solution measurement of acidity describing a solution that is neither an acid nor a base substance that has a pH of 7 A metal made of 2 or more solids 2 or more substances mixed together but not chemically combined Homogenous – particles/ substances are evenly ...
Lesson 2a - Freeman Public Schools
... Compound—two or more different atoms combined chemically ...
... Compound—two or more different atoms combined chemically ...
Redox I
... If the redox reaction takes place in BASIC solution, use steps 1-6 (as before) to balance the equation as if it took place in acidic solution. Then perform one more step: Step 7. (ONLY for redox reactions taking place in basic solution!) Add OH- to BOTH sides of the equation to cancel all of the H+, ...
... If the redox reaction takes place in BASIC solution, use steps 1-6 (as before) to balance the equation as if it took place in acidic solution. Then perform one more step: Step 7. (ONLY for redox reactions taking place in basic solution!) Add OH- to BOTH sides of the equation to cancel all of the H+, ...
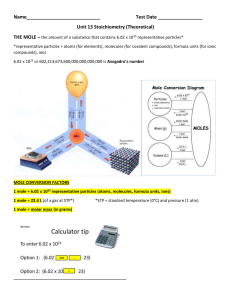
Unit 13 Stoichiometry (Theoretical)
... Ionic compounds o In 1 mole of Ca(NO3)2 there are 1 mole of calcium ions and 2 moles of nitrate ions o In 1 mole of K2O, the ion ratio is 2 mole K+1 ions: 1 mole of O-2 ions ...
... Ionic compounds o In 1 mole of Ca(NO3)2 there are 1 mole of calcium ions and 2 moles of nitrate ions o In 1 mole of K2O, the ion ratio is 2 mole K+1 ions: 1 mole of O-2 ions ...
Aberration-Corrected Analytical Electron Microscopy of Transition Metal Nitride and Silicon Nitride Multilayers
... contributed to the research of adding an element to make a ternary nitride. One such element often applied, which is found in both papers presented in this work, is silicon that is more or less immiscible in TmN. For very small amounts of Si addition a single-phase film may be produced, but often ju ...
... contributed to the research of adding an element to make a ternary nitride. One such element often applied, which is found in both papers presented in this work, is silicon that is more or less immiscible in TmN. For very small amounts of Si addition a single-phase film may be produced, but often ju ...
Challenging chemical misconceptions in the classroom?
... So - once the teacher is aware that an alternative conceptions is operating - it is necessary to make the pupils aware that they have not understood the scientific idea. Before the teacher can try and make the material make (the intended) sense for the pupil, they have to persuade them that their cu ...
... So - once the teacher is aware that an alternative conceptions is operating - it is necessary to make the pupils aware that they have not understood the scientific idea. Before the teacher can try and make the material make (the intended) sense for the pupil, they have to persuade them that their cu ...
(III) From Aqueous Solutions with Sodium Dodecyl Sulfate
... raising the products of flotation and facilitating its removal, but in some cases, for example, by the extraction of organic ions the excess foam and slow its destruction can be only a hindrance. An important property of the method is the high degree of selectivity. In flotation process [9] using su ...
... raising the products of flotation and facilitating its removal, but in some cases, for example, by the extraction of organic ions the excess foam and slow its destruction can be only a hindrance. An important property of the method is the high degree of selectivity. In flotation process [9] using su ...
Spectra and Atomic Structure
... Absorption can boost an electron to the second (or higher) excited state Two ways to decay: 1. to ground state 2. cascade one orbital at a time ...
... Absorption can boost an electron to the second (or higher) excited state Two ways to decay: 1. to ground state 2. cascade one orbital at a time ...
LESSON ASSIGNMENT LESSON 2 Elements of Chemical Change
... b. Write the correct formulas for any compounds and check for diatomic molecules. (Some elements never exist as single atoms but only as diatomic molecules. These elements can be identified from their names, which end in -gen or -ine. The common diatomic molecules are hydrogen (H2), nitrogen (N2), o ...
... b. Write the correct formulas for any compounds and check for diatomic molecules. (Some elements never exist as single atoms but only as diatomic molecules. These elements can be identified from their names, which end in -gen or -ine. The common diatomic molecules are hydrogen (H2), nitrogen (N2), o ...
5H2O → CuSO4 + 5H2O(g)
... When iron rusts, it loses electrons to form a cation, oxygen gain electrons to form an anion: 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) Use oxidation number rules to determine gain and loss of electrons. Oxidation numbers are assigned as if elements in compounds completely transferred electrons (like in ionic ...
... When iron rusts, it loses electrons to form a cation, oxygen gain electrons to form an anion: 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) Use oxidation number rules to determine gain and loss of electrons. Oxidation numbers are assigned as if elements in compounds completely transferred electrons (like in ionic ...
Balancing Reaction Equations Oxidation State Reduction
... Oxidation: Loss of electrons from an element. Oxidation number increases Reduction: Gain of electrons by an element. Oxidation number decreases ...
... Oxidation: Loss of electrons from an element. Oxidation number increases Reduction: Gain of electrons by an element. Oxidation number decreases ...
Cold Fusion By Plasma Electrolysis of Water
... – there were no conditions for the formation of the photons in the process being considered, and mass mF , which failed to be formed as a particle, “was dissolved” in the ether. Which variant is closer to the truth [6] ? There is no exact answer, but it is known that the Japanese scientists register ...
... – there were no conditions for the formation of the photons in the process being considered, and mass mF , which failed to be formed as a particle, “was dissolved” in the ether. Which variant is closer to the truth [6] ? There is no exact answer, but it is known that the Japanese scientists register ...
Acid Base PPT - mvhs
... The pH Scale The pH of a solution is defined as the negative of the common logarithm of the hydronium ion concentration. pH= -log [H3O+] The pOH of a solution is defined as the negative of the common logarithm of the hydroxide ion concentration. ...
... The pH Scale The pH of a solution is defined as the negative of the common logarithm of the hydronium ion concentration. pH= -log [H3O+] The pOH of a solution is defined as the negative of the common logarithm of the hydroxide ion concentration. ...
Introduction
... When iron rusts, it loses electrons to form a cation, oxygen gain electrons to form an anion: 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) Use oxidation number rules to determine gain and loss of electrons. Oxidation numbers are assigned as if elements in compounds completely transferred electrons (like in ionic ...
... When iron rusts, it loses electrons to form a cation, oxygen gain electrons to form an anion: 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) Use oxidation number rules to determine gain and loss of electrons. Oxidation numbers are assigned as if elements in compounds completely transferred electrons (like in ionic ...
CHEMISTRY
... much ZnCl2 is formed, it is necessary to divide 10 / mw of Zinc (65,37) to have the number of moles (= 0,153). The same number of moles of ZnCl2 (see reaction coefficients). The mass of ZnCl2 will be = number of moles (0,153) x mm of the salt (134,27) = 20,5 g In the above reaction H2 (gas) was form ...
... much ZnCl2 is formed, it is necessary to divide 10 / mw of Zinc (65,37) to have the number of moles (= 0,153). The same number of moles of ZnCl2 (see reaction coefficients). The mass of ZnCl2 will be = number of moles (0,153) x mm of the salt (134,27) = 20,5 g In the above reaction H2 (gas) was form ...
Course __Chemistry Sept Oct Nov Dec Jan Feb March April May June
... provide models of atoms and molecules. B2. Atoms combine to form molecules or formula units by sharing electrons to form covalent or metallic bonds (respectively), or by exchanging electrons to form ionic bonds. B3. Chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2 and ...
... provide models of atoms and molecules. B2. Atoms combine to form molecules or formula units by sharing electrons to form covalent or metallic bonds (respectively), or by exchanging electrons to form ionic bonds. B3. Chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2 and ...
practice final examination
... 10. Answer true or false for each of the following questions below (circle your choice): a) ...
... 10. Answer true or false for each of the following questions below (circle your choice): a) ...
AP Chemistry Summer Assignment 2016
... Avogadro’s Number, the molar volume of a gas measured at STP, and the meaning of the term STP. Solve the following problems and check your answers. You must be able to use units correctly in your solutions. Dimensional analysis is recommended for solving these types of problems. 1.) Given 2.50 grams ...
... Avogadro’s Number, the molar volume of a gas measured at STP, and the meaning of the term STP. Solve the following problems and check your answers. You must be able to use units correctly in your solutions. Dimensional analysis is recommended for solving these types of problems. 1.) Given 2.50 grams ...
Document
... Jan Baptista van Helmont (1579–1644) first measured the mass of a young willow tree and, separately, the mass of a bucket of soil and then planted the tree in the bucket. After five years, he found that the tree had gained 75 kg in mass even though the soil had lost only 0.057 kg. He had added only ...
... Jan Baptista van Helmont (1579–1644) first measured the mass of a young willow tree and, separately, the mass of a bucket of soil and then planted the tree in the bucket. After five years, he found that the tree had gained 75 kg in mass even though the soil had lost only 0.057 kg. He had added only ...
Lecture 5 – Chemical Reactions
... In this reaction the oxygen is taking away 3 electrons from each iron to produce Fe3+ ions. However, even in covalent compounds, where the valence electrons are shared, the sharing is not equal when oxygen is involved. i. It is useful to consider that the oxygen is taking the electrons away from the ...
... In this reaction the oxygen is taking away 3 electrons from each iron to produce Fe3+ ions. However, even in covalent compounds, where the valence electrons are shared, the sharing is not equal when oxygen is involved. i. It is useful to consider that the oxygen is taking the electrons away from the ...
NOTE Mixed-Ligand Complexes of Cu2+, Ni2+, Co2+, Zn2+ with 2,2
... It has been observed that primary complex curve C diverges from the Bipy Curve B in the pH range at about 2 and onwards. The metal-Bipy 1:1 complex formation is complete at pH 4.0 and it forms hydroxo complex at pH about 8.0. Primary complex curve C and ternary complex curve E however overlap at low ...
... It has been observed that primary complex curve C diverges from the Bipy Curve B in the pH range at about 2 and onwards. The metal-Bipy 1:1 complex formation is complete at pH 4.0 and it forms hydroxo complex at pH about 8.0. Primary complex curve C and ternary complex curve E however overlap at low ...
High School Knowledge Exam – Study Guide
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
Shifting Equilibrium
... Reversible reactions are exothermic in one direction and endothermic in the other. Remember, equilibrium constants are for a given temperature, because changing the temperature changes the relative amounts of reactants and products. Increasing the temperature is, in effect, the addition of energy in ...
... Reversible reactions are exothermic in one direction and endothermic in the other. Remember, equilibrium constants are for a given temperature, because changing the temperature changes the relative amounts of reactants and products. Increasing the temperature is, in effect, the addition of energy in ...