Building the sense of math in physics activities
... 1. In the figure are shown four cases with different arrangements of charge. Each charge has the same magnitude, but some are + and some are -. All distances are to the same scale. We were considering the force exerted on a positive charge placed at the points, P. 1.1 If the positive charge, P, is r ...
... 1. In the figure are shown four cases with different arrangements of charge. Each charge has the same magnitude, but some are + and some are -. All distances are to the same scale. We were considering the force exerted on a positive charge placed at the points, P. 1.1 If the positive charge, P, is r ...
jyvaskla2 - School of Chemistry
... very much like the balls and spheres of molecular models !!! The simple binary hydrides of the second period elements show that the relative volumes of space associated with each element is determined by their relative electronegativities. Surfaces are truncated at 0.001 au. ...
... very much like the balls and spheres of molecular models !!! The simple binary hydrides of the second period elements show that the relative volumes of space associated with each element is determined by their relative electronegativities. Surfaces are truncated at 0.001 au. ...
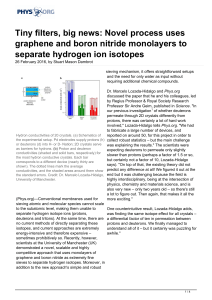
Tiny filters, big news: Novel process uses graphene and
... reported on around 50, for this project in order to the experimental setup. Pd electrodes supply protons (h) collect robust statistics – but the main challenge or deuterons (d) into H- or D- Nation; 2D crystals serve was explaining the results." The scientists were as barriers for hydrons. (b) Proto ...
... reported on around 50, for this project in order to the experimental setup. Pd electrodes supply protons (h) collect robust statistics – but the main challenge or deuterons (d) into H- or D- Nation; 2D crystals serve was explaining the results." The scientists were as barriers for hydrons. (b) Proto ...
Modeling the extraction of sputtered metal from Linköping University Post Print
... 2. The experimental setup We here apply the model to the setup for copper nanoparticle synthesis shown in figure 2a, where material is sputtered from a hollow cathode. Nanoparticles are formed in an expansion zone outside the hollow cathode exit (see figure 2a) and collected on substrates. The proc ...
... 2. The experimental setup We here apply the model to the setup for copper nanoparticle synthesis shown in figure 2a, where material is sputtered from a hollow cathode. Nanoparticles are formed in an expansion zone outside the hollow cathode exit (see figure 2a) and collected on substrates. The proc ...
L-Cysteine as a Chiral Linker in Lanthanide–Cucurbit[6]uril
... Synthesis of complexes 1–3. CB6·5H2O (11 mg, 0.01 mmol), Ln(NO3)3·xH2O (Nd: x = 6, 44 mg, 0.10 mmol; Eu: x = 5, 43 mg, 0.10 mmol; Tb: x = 6, 45 mg, 0.10 mmol) and L-cysteine (24 mg, 0.20 mmol) were dissolved in demineralized water (1.5 mL) upon gentle heating. The solutions were then left to evapora ...
... Synthesis of complexes 1–3. CB6·5H2O (11 mg, 0.01 mmol), Ln(NO3)3·xH2O (Nd: x = 6, 44 mg, 0.10 mmol; Eu: x = 5, 43 mg, 0.10 mmol; Tb: x = 6, 45 mg, 0.10 mmol) and L-cysteine (24 mg, 0.20 mmol) were dissolved in demineralized water (1.5 mL) upon gentle heating. The solutions were then left to evapora ...
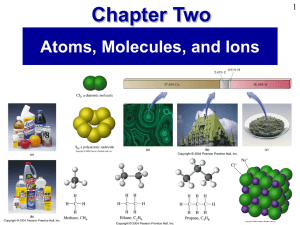
Prentice Hall Ch 02 Atoms Molecules Ions
... Ions and Ionic Compounds • An atom that either gains or loses electron(s) is an ion. • There is no change in the number of protons or neutrons in the nucleus of the atom. • Cation – has a positive charge from loss of electron(s). • Anion – has a negative charge from gain of electron(s). ...
... Ions and Ionic Compounds • An atom that either gains or loses electron(s) is an ion. • There is no change in the number of protons or neutrons in the nucleus of the atom. • Cation – has a positive charge from loss of electron(s). • Anion – has a negative charge from gain of electron(s). ...
Diagnostic research of highly ionized plasma generated by an ECR
... One of the research directions of the ATOMKI-ECRIS is the systematic investigation of the con"ned plasma. In our laboratory we have the instrumentation background for two plasma research methods: X-ray and visible light diagnostics is possible outside the plasma chamber and the plasma can be investi ...
... One of the research directions of the ATOMKI-ECRIS is the systematic investigation of the con"ned plasma. In our laboratory we have the instrumentation background for two plasma research methods: X-ray and visible light diagnostics is possible outside the plasma chamber and the plasma can be investi ...
Chemistry Olympiad Support Booklet
... Every year the Royal Society of Chemistry (RSC) organises the selection of the UK team for the International Chemistry Olympiad (IChO). The IChO has been running for 40 years, and the UK has been involved since 1983. Next year, in July 2009, the UK will be hosting the competition, and almost 300 stu ...
... Every year the Royal Society of Chemistry (RSC) organises the selection of the UK team for the International Chemistry Olympiad (IChO). The IChO has been running for 40 years, and the UK has been involved since 1983. Next year, in July 2009, the UK will be hosting the competition, and almost 300 stu ...
Summer Assignment: Some Review / Basic Prep
... moving electrolytes (+ and -) allow for the conductance of an electrical current Yes: The fused compound (melted or liquefied phase) has had the ionic bond(s) broken and thus electrolytes have been produced. No: There are no free moving electrolytes. Really, only metals conduct electricity as a soli ...
... moving electrolytes (+ and -) allow for the conductance of an electrical current Yes: The fused compound (melted or liquefied phase) has had the ionic bond(s) broken and thus electrolytes have been produced. No: There are no free moving electrolytes. Really, only metals conduct electricity as a soli ...
Chemistry II Exams and Keys 2014 Season
... 6. A bomb calorimeter is calibrated by combusting 1.558 g of benzoic acid (MW = 122.2 g/mol) in the chamber. The temperature of the water is increased by 2.34 K. The enthalpy of combustion of benzoic acid is -3230 kJ/mol. After determining the calorimetric constant, the very same bomb calorimeter is ...
... 6. A bomb calorimeter is calibrated by combusting 1.558 g of benzoic acid (MW = 122.2 g/mol) in the chamber. The temperature of the water is increased by 2.34 K. The enthalpy of combustion of benzoic acid is -3230 kJ/mol. After determining the calorimetric constant, the very same bomb calorimeter is ...
AP Chemistry Review Preparing for the AP
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
PHYSICAL SETTING CHEMISTRY
... (3) gain electrons and have a decrease in oxidation number (4) gain electrons and have an increase in oxidation number ...
... (3) gain electrons and have a decrease in oxidation number (4) gain electrons and have an increase in oxidation number ...
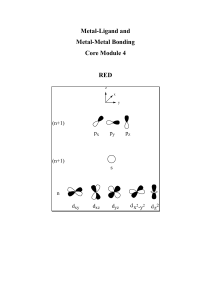
Metal-Ligand and Metal-Metal Bonding Lecture Notes
... very similar. E.g. Co(III) (0.55), Rh(III) (0.67), Ir(III) (0.68). This has repercussions in metalligand bonding and hence chemical properties. In general when descending a group the 1st row transition metal is distinct in terms of its bonding and properties from the 2nd and 3rd row metals. 3. Varie ...
... very similar. E.g. Co(III) (0.55), Rh(III) (0.67), Ir(III) (0.68). This has repercussions in metalligand bonding and hence chemical properties. In general when descending a group the 1st row transition metal is distinct in terms of its bonding and properties from the 2nd and 3rd row metals. 3. Varie ...
formula writing and nomenclature of inorganic compounds
... share electrons in forming a chemical bond. The number of electrons that an atom loses, gains, or shares when it bonds with another atom is known as the oxidation number of the atom. Elements which lose electrons in a chemical reaction, or which have electrons which are shared with another element d ...
... share electrons in forming a chemical bond. The number of electrons that an atom loses, gains, or shares when it bonds with another atom is known as the oxidation number of the atom. Elements which lose electrons in a chemical reaction, or which have electrons which are shared with another element d ...
GHW - Louisiana Tech University
... of eggs easier. Since atoms are so small, we need large number of them to make it physically observable and able to weigh in gram quantities. Gram Mole and the Avogadro's Number The gram mole is the grams of any chemical substance using the value atomic mass obtained from the periodic table. E.g. fo ...
... of eggs easier. Since atoms are so small, we need large number of them to make it physically observable and able to weigh in gram quantities. Gram Mole and the Avogadro's Number The gram mole is the grams of any chemical substance using the value atomic mass obtained from the periodic table. E.g. fo ...
The 2016 AP Chemistry Exam will be Monday
... passed since you have had a chemistry course, it is imperative that you come to class the first day with Sections A and B completed. This summer assignment is not optional, and completing the assignment in a thorough and focused manner will contribute to a student’s success in this course and on the ...
... passed since you have had a chemistry course, it is imperative that you come to class the first day with Sections A and B completed. This summer assignment is not optional, and completing the assignment in a thorough and focused manner will contribute to a student’s success in this course and on the ...
Module 3: Defects, Diffusion and Conduction in Ceramics
... called as diffusion. Diffusivity of species in the materials is also related to their physical properties such as electrical conductivity and mobility via Nernst-Einestein relation which we shall derive. As we shall also see, the conductivity in ceramics is a sum of ionic and electronic conductivity ...
... called as diffusion. Diffusivity of species in the materials is also related to their physical properties such as electrical conductivity and mobility via Nernst-Einestein relation which we shall derive. As we shall also see, the conductivity in ceramics is a sum of ionic and electronic conductivity ...
Reduction
... Precipitation has the effect of significantly changing the half-cell potential for the reduction of Mn(III). In solutions in which [OH]= 1M, Mn(III) is stabilized with respect to reduction to Mn(II) as the value of Eo[OH]=1 for equation 8.35 illustrates. Compare this with ...
... Precipitation has the effect of significantly changing the half-cell potential for the reduction of Mn(III). In solutions in which [OH]= 1M, Mn(III) is stabilized with respect to reduction to Mn(II) as the value of Eo[OH]=1 for equation 8.35 illustrates. Compare this with ...
PHYS 4740 Lecture notes 1
... forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid. The vacancies are then free to move about as their own entities. Normally these defects will lead to a decre ...
... forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid. The vacancies are then free to move about as their own entities. Normally these defects will lead to a decre ...
Chapter 2_Application Problems
... • Water is composed of many identical molecules that have one oxygen atom and two hydrogen atoms – correct; according to Dalton, atoms combine together in compounds in small whole-number ratios, so that you could describe a compound by describing the number of atoms of each element in a molecule. He ...
... • Water is composed of many identical molecules that have one oxygen atom and two hydrogen atoms – correct; according to Dalton, atoms combine together in compounds in small whole-number ratios, so that you could describe a compound by describing the number of atoms of each element in a molecule. He ...
幻灯片 1
... opposed by electrostatic repulsion between protons. Electrostatic repulsion force: among the protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. ...
... opposed by electrostatic repulsion between protons. Electrostatic repulsion force: among the protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. ...
2017 Chemistry Exam Review Compounds and Reactions 1. Know
... 36. Be able to tell from their chemical formulas which of two fuels produces more energy per mole and why. Solutions 37. Define solution in terms of solute and solvent? What is the solvent in an aqueous solution? 38. Describe and show (with electronegativity values and an electron dot diagram) the p ...
... 36. Be able to tell from their chemical formulas which of two fuels produces more energy per mole and why. Solutions 37. Define solution in terms of solute and solvent? What is the solvent in an aqueous solution? 38. Describe and show (with electronegativity values and an electron dot diagram) the p ...
Supplementary Exercise 1B Topic 5
... In the electrochemical series, the position of calcium is higher than that of sodium. The order is different from that in the reactivity series. This is because calcium atom loses electrons more readily in cell reactions than in reaction with air, water and dilute acids. ...
... In the electrochemical series, the position of calcium is higher than that of sodium. The order is different from that in the reactivity series. This is because calcium atom loses electrons more readily in cell reactions than in reaction with air, water and dilute acids. ...
Chem 111 2:30p section Final Exam
... 39a. In ionizing elemental sodium to Na , from which orbital is an electron removed? 1) 1s ...
... 39a. In ionizing elemental sodium to Na , from which orbital is an electron removed? 1) 1s ...
Preview to Mole Activity #2 preview_to_mole_activity_21
... A long time ago chemists discovered what you just discovered by answering question 8. If they were talking about the mass of one atom of an element they talked about its mass in amu’s. This was not very helpful as most often they were dealing with many more atoms than just one or two. What they foun ...
... A long time ago chemists discovered what you just discovered by answering question 8. If they were talking about the mass of one atom of an element they talked about its mass in amu’s. This was not very helpful as most often they were dealing with many more atoms than just one or two. What they foun ...

![L-Cysteine as a Chiral Linker in Lanthanide–Cucurbit[6]uril](http://s1.studyres.com/store/data/002388013_1-9387091f243aaed6c7cd4b5e1425ce91-300x300.png)