halogen compounds organic chemistry
... chloride or cuprous bromide dissolved in corresponding halogen acids. Chloro and bromoarenes are formed. Diazonium salts required for this purpose are prepared by treating ice-cold solution of aniline in excess of dilute HCl with an aqueous solution of sodium nitrite at low temperature (0-5oC). This ...
... chloride or cuprous bromide dissolved in corresponding halogen acids. Chloro and bromoarenes are formed. Diazonium salts required for this purpose are prepared by treating ice-cold solution of aniline in excess of dilute HCl with an aqueous solution of sodium nitrite at low temperature (0-5oC). This ...
CHEMISTRY
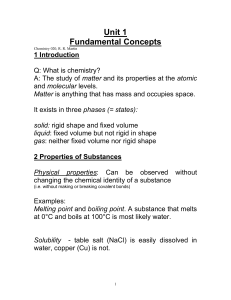
... Homogeneous matter has the same physical and chemical properties throughout it. In other words, all the stuff that makes up homogeneous matter acts pretty much the same way. Homogeneous matter is called a “substance.” Heterogeneous matter is a mixture of substances, like a rock formed from fragments ...
... Homogeneous matter has the same physical and chemical properties throughout it. In other words, all the stuff that makes up homogeneous matter acts pretty much the same way. Homogeneous matter is called a “substance.” Heterogeneous matter is a mixture of substances, like a rock formed from fragments ...
Introductory Review
... For ionic compounds, e.g. sodium chloride, the formula shows the ratio of elements that form the compound. Solid sodium chloride consists of a collection of positively charged sodium ions and negatively charged chloride ions in a three-dimensional structure. You cannot say which sodium ion is assoc ...
... For ionic compounds, e.g. sodium chloride, the formula shows the ratio of elements that form the compound. Solid sodium chloride consists of a collection of positively charged sodium ions and negatively charged chloride ions in a three-dimensional structure. You cannot say which sodium ion is assoc ...
Chapter 19
... Note that chlorine “takes” electrons from bromide ions to become chloride ions. When the two bromide ions lose electrons, the two bromine atoms form a covalent bond with each other to produce Br 2 molecules. The formation of the covalent bond by sharing of electrons is also an oxidation-reduction re ...
... Note that chlorine “takes” electrons from bromide ions to become chloride ions. When the two bromide ions lose electrons, the two bromine atoms form a covalent bond with each other to produce Br 2 molecules. The formation of the covalent bond by sharing of electrons is also an oxidation-reduction re ...
Alfred Werner: Father of Coordination Chemistry.
... P(C6H5)3 + 2 Na0 → Na+P(C6H5)2- + Na+C6H52 NaP(C6H5)2 + ClCH2CH2Cl → (C6H5)2PCH2CH2P(C6H5)2 + 2 NaCl ...
... P(C6H5)3 + 2 Na0 → Na+P(C6H5)2- + Na+C6H52 NaP(C6H5)2 + ClCH2CH2Cl → (C6H5)2PCH2CH2P(C6H5)2 + 2 NaCl ...
C:\usb key\sch3u\unit 1\chapter 2 test answers.wpd
... 8) Identify one compound from question 7 that would have each of the following inter-particle forces. Chose a different compound for each force. a) ionic bonds b) London forces c) Hydrogen bonds d) dipole/dipole forces ...
... 8) Identify one compound from question 7 that would have each of the following inter-particle forces. Chose a different compound for each force. a) ionic bonds b) London forces c) Hydrogen bonds d) dipole/dipole forces ...
Page 1 MISE - Physical Basis of Chemistry First Set of Problems
... allowed scientists to investigate the sub-structure and size of atoms. The atom was not homogeneous, i.e., not of uniform density. Most of the mass of an atom was contained in a very small volume – termed the nucleus. This nucleus had a net positive charge. The remainder of the atom was mostly “empt ...
... allowed scientists to investigate the sub-structure and size of atoms. The atom was not homogeneous, i.e., not of uniform density. Most of the mass of an atom was contained in a very small volume – termed the nucleus. This nucleus had a net positive charge. The remainder of the atom was mostly “empt ...
Skill Practice 1
... 1. On each of the phase diagrams label the triple point (TP) and the solid, liquid and vapor states. 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allo ...
... 1. On each of the phase diagrams label the triple point (TP) and the solid, liquid and vapor states. 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allo ...
Novel Example of a Chain Structure Formed by 1,4
... linked by a weak and hindered base such as 1,4-dioxane (see Figure 2). One chain is separated from the neighbor chains [10.240(1) Å] by a network of 1,4-dioxane molecules held by a network of hydrogen bonds. The large distance between parallel chains precludes magnetic interactions between them. Mod ...
... linked by a weak and hindered base such as 1,4-dioxane (see Figure 2). One chain is separated from the neighbor chains [10.240(1) Å] by a network of 1,4-dioxane molecules held by a network of hydrogen bonds. The large distance between parallel chains precludes magnetic interactions between them. Mod ...
Descriptive Chemistry of Elements p
... central atom refers to the number of atoms attached to the central atom. Coordination number of carbon atom in CO2 and CO3 can be considered as 2 and 3, respectively. Normally the maximum coordination number of carbon is four as the maximum number of electrons in the valence shell is eight. Howeve ...
... central atom refers to the number of atoms attached to the central atom. Coordination number of carbon atom in CO2 and CO3 can be considered as 2 and 3, respectively. Normally the maximum coordination number of carbon is four as the maximum number of electrons in the valence shell is eight. Howeve ...
231. - Department of Chemistry
... measurements since it is highly suitable for the investigation of the ligation of cations with weakly bonded ligands due to the relatively high helium pressure of the bath gas (0.35 Torr). The high number of collisions with helium atoms that result from the presence of bath gas serve both to thermal ...
... measurements since it is highly suitable for the investigation of the ligation of cations with weakly bonded ligands due to the relatively high helium pressure of the bath gas (0.35 Torr). The high number of collisions with helium atoms that result from the presence of bath gas serve both to thermal ...
Experiment 4 - Macalester College
... The “placement” of the nonbonding pairs was straightforward in the previous examples, since each site about the central atom was equivalent. This is not the case with trigonal bipyramidal molecules. The sites that are 120o apart are called “equatorial,” while the sites that are 90o relative to the e ...
... The “placement” of the nonbonding pairs was straightforward in the previous examples, since each site about the central atom was equivalent. This is not the case with trigonal bipyramidal molecules. The sites that are 120o apart are called “equatorial,” while the sites that are 90o relative to the e ...
Questions and Solutions
... An old barometer hanging on the wall of a mountain hut has a reading of 25.5 inches of mercury. If 1 inch of mercury equals 0.0334 atm (atmoshperes) and 1 atm = 101.3 kPa and 1 kPa = 7.50 torr. What is the pressure reading of the barometer in torr? 647 torr ...
... An old barometer hanging on the wall of a mountain hut has a reading of 25.5 inches of mercury. If 1 inch of mercury equals 0.0334 atm (atmoshperes) and 1 atm = 101.3 kPa and 1 kPa = 7.50 torr. What is the pressure reading of the barometer in torr? 647 torr ...
A Review of High School Chemistry
... FORMULA WEIGHT (MOLECULAR WEIGHT) To this point, we’ve dealt with moles of atoms. Now we deal with MOLES of MOLECULES by introducing the concept of formula or molecular weight (these are the same if dealing with molecules. If dealing with ionic materials, it is more appropriate to describe a formula ...
... FORMULA WEIGHT (MOLECULAR WEIGHT) To this point, we’ve dealt with moles of atoms. Now we deal with MOLES of MOLECULES by introducing the concept of formula or molecular weight (these are the same if dealing with molecules. If dealing with ionic materials, it is more appropriate to describe a formula ...
Chemical bonding
... bonds. The orbital dipole decreases the effect of the resultant N-F bond moments which reduces dipole moment of NF3. 7) Ans: For the resonance structures of CO32- and CO2 refer to page no 106( Part-1) 8) Ans: a) Deviation in the shape of the molecule b) Alterations in the bond angle in the molecule. ...
... bonds. The orbital dipole decreases the effect of the resultant N-F bond moments which reduces dipole moment of NF3. 7) Ans: For the resonance structures of CO32- and CO2 refer to page no 106( Part-1) 8) Ans: a) Deviation in the shape of the molecule b) Alterations in the bond angle in the molecule. ...
1 - Cathedral High School
... 3.2.1 Describe and explain the periodic trends in atomic radii, ionic radii, ionization energies, electronegativity and melting points for the alkali metals (Li Cs), halogens (F I) and period 3 elements (Na Ar). Cross reference with topics 2, 4 and 5. Data for all these properties are listed i ...
... 3.2.1 Describe and explain the periodic trends in atomic radii, ionic radii, ionization energies, electronegativity and melting points for the alkali metals (Li Cs), halogens (F I) and period 3 elements (Na Ar). Cross reference with topics 2, 4 and 5. Data for all these properties are listed i ...
Answers - Benjamin
... If there is any manganese in a sample of steel a pink colour will appear when the steel sample has been dissolved in nitric acid and then oxidised with potassium iodate(VII). ...
... If there is any manganese in a sample of steel a pink colour will appear when the steel sample has been dissolved in nitric acid and then oxidised with potassium iodate(VII). ...
PowerPoint 演示文稿
... brought into same phase. However they do not dissolve glass, polyethylene, or Teflon. High solubility usually implies small reactor volumes in the final process. They are immiscible with a number of organic solvents and provide a non-aqueous, polar alternative for two phase systems, this has been us ...
... brought into same phase. However they do not dissolve glass, polyethylene, or Teflon. High solubility usually implies small reactor volumes in the final process. They are immiscible with a number of organic solvents and provide a non-aqueous, polar alternative for two phase systems, this has been us ...
NCEA Level 3 Chemistry (91392) 2015
... • One correct equation. OR Four species identified. • Correct order, all species. • Correct process. ...
... • One correct equation. OR Four species identified. • Correct order, all species. • Correct process. ...
The Voltaic Origins of Helmholtz`s Physics of Ions
... electromotive force. In particular, the smallest electromotive force was sufficient to start the current. This contradicted the received idea that the decomposition of the electrolytic substance required a minimal electromotive force. In 1857, Rudolf Clausius solved the paradox by means of his kinet ...
... electromotive force. In particular, the smallest electromotive force was sufficient to start the current. This contradicted the received idea that the decomposition of the electrolytic substance required a minimal electromotive force. In 1857, Rudolf Clausius solved the paradox by means of his kinet ...
NSCC Chem 121 chapter5
... that are dissolved in water. Ionic compounds and some polar covalent compounds break apart (dissociate) when they dissolve in water and form ions. ...
... that are dissolved in water. Ionic compounds and some polar covalent compounds break apart (dissociate) when they dissolve in water and form ions. ...
Process- och Komponentteknologi FFF110
... MOS structures are the fundamental building blocks for capacitors, transistors, CCDs and memories. MOSFET idea: A voltage applied to the gate should change the number of mobile carriers in the Si. In the ideal MOS no charges exit during bias other than those in the semiconductor. In a real, unwanted ...
... MOS structures are the fundamental building blocks for capacitors, transistors, CCDs and memories. MOSFET idea: A voltage applied to the gate should change the number of mobile carriers in the Si. In the ideal MOS no charges exit during bias other than those in the semiconductor. In a real, unwanted ...
Chemistry for the Pharmacy Technician
... Remember that electrolytes carry an electrical charge in solution. In a stable compound, the number of positive electrical charges must equal the number of negative charges. Thus salt is a sodium chloride compound consisting of both sodium (positive electrical charge) and chloride (negative electric ...
... Remember that electrolytes carry an electrical charge in solution. In a stable compound, the number of positive electrical charges must equal the number of negative charges. Thus salt is a sodium chloride compound consisting of both sodium (positive electrical charge) and chloride (negative electric ...
Chemistry 11 Exam 1 Spring 2006 When answering questions be
... 12. Write the electron configuration (spdf) for iron (Fe). Then show the shorthand notation based on noble gas configurations. 1s2 2s2 2p6 3s2 3p6 4s2 3d6 = [Ar] 4s2 3d6 13. The atomic radii for the first four alkali metals are shown below. Explain this pattern. Moving down the periodic table the o ...
... 12. Write the electron configuration (spdf) for iron (Fe). Then show the shorthand notation based on noble gas configurations. 1s2 2s2 2p6 3s2 3p6 4s2 3d6 = [Ar] 4s2 3d6 13. The atomic radii for the first four alkali metals are shown below. Explain this pattern. Moving down the periodic table the o ...