elements of chemistry unit
... For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation number. For Al2O3: Oxygen is a group 16 element, so each oxygen atom has a – 2 oxidation number. Since there are 3 oxygen atoms in Al2O3, the O3 atoms have a combined – 6 o ...
... For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation number. For Al2O3: Oxygen is a group 16 element, so each oxygen atom has a – 2 oxidation number. Since there are 3 oxygen atoms in Al2O3, the O3 atoms have a combined – 6 o ...
111 Exam I Outline
... Chromium metal is reacted with copper (II) chloride Key: You must have a balanced equation!! How many grams of chromic chloride reacts with 6.0 mole Cr? ...
... Chromium metal is reacted with copper (II) chloride Key: You must have a balanced equation!! How many grams of chromic chloride reacts with 6.0 mole Cr? ...
Atoms and bonds in molecules and chemical explanations
... main features of complex atomic systems without too much computation. This prescription has been anticipated 2 years before Dirac’s paper by the calculations of the dihydrogen molecule of Heitler and London (1927) on the one hand and of Condon (1927) on the other hand. 2 Heitler and London’s paper w ...
... main features of complex atomic systems without too much computation. This prescription has been anticipated 2 years before Dirac’s paper by the calculations of the dihydrogen molecule of Heitler and London (1927) on the one hand and of Condon (1927) on the other hand. 2 Heitler and London’s paper w ...
Chapter 18 - WordPress.com
... Electrochemistry is the study of redox reactions that produce or require an electric current. The conversion between chemical energy and electrical energy is carried out in an electrochemical cell. Spontaneous redox reactions take place in a voltaic cell. aka galvanic cells Nonspontaneous redox re ...
... Electrochemistry is the study of redox reactions that produce or require an electric current. The conversion between chemical energy and electrical energy is carried out in an electrochemical cell. Spontaneous redox reactions take place in a voltaic cell. aka galvanic cells Nonspontaneous redox re ...
111 Exam I Outline
... Chromium metal is reacted with copper (II) chloride Key: You must have a balanced equation!! How many grams of chromic chloride reacts with 6.0 mole Cr? ...
... Chromium metal is reacted with copper (II) chloride Key: You must have a balanced equation!! How many grams of chromic chloride reacts with 6.0 mole Cr? ...
HEFAT2014 10 International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics
... can be presented in bipolar semiconductors when the total electrical current is absent. Therefore, the energy can be transferred not only by means of heat conductivity but also by means of electrons' and holes' transports since they carry the energy. The process of an appearance of these electrical ...
... can be presented in bipolar semiconductors when the total electrical current is absent. Therefore, the energy can be transferred not only by means of heat conductivity but also by means of electrons' and holes' transports since they carry the energy. The process of an appearance of these electrical ...
Chapter 6 Chemical reactions Classification And Mass Relationships
... • To know if the precipitation reaction will occur, we must first know the solubility of the products formed. • If the solubility of the product is low then the product is likely to precipitate. • If the product has a high solubility then the product is not likely to form a precipitate. ...
... • To know if the precipitation reaction will occur, we must first know the solubility of the products formed. • If the solubility of the product is low then the product is likely to precipitate. • If the product has a high solubility then the product is not likely to form a precipitate. ...
Revisiting the role of Calcium and Gypsum for Sodic
... Outer-sphere complexes are created when at least one water molecule is present between the ion or molecule and the SFG. These complexes have the following characteristics: ...
... Outer-sphere complexes are created when at least one water molecule is present between the ion or molecule and the SFG. These complexes have the following characteristics: ...
Section 8.10 Lewis Structures
... 3. Atoms usually have noble gas configurations. Arrange the remaining electrons to satisfy the octet rule (or duet rule for hydrogen). Examples: H2O, PBr3, and HCN ...
... 3. Atoms usually have noble gas configurations. Arrange the remaining electrons to satisfy the octet rule (or duet rule for hydrogen). Examples: H2O, PBr3, and HCN ...
Metal-Ligand and Metal-Metal Bonding Core Module 4 RED
... very similar. E.g. Co(III) (0.55), Rh(III) (0.67), Ir(III) (0.68). This has repercussions in metalligand bonding and hence chemical properties. In general when descending a group the 1st row transition metal is distinct in terms of its bonding and properties from the 2nd and 3rd row metals. 3. Varie ...
... very similar. E.g. Co(III) (0.55), Rh(III) (0.67), Ir(III) (0.68). This has repercussions in metalligand bonding and hence chemical properties. In general when descending a group the 1st row transition metal is distinct in terms of its bonding and properties from the 2nd and 3rd row metals. 3. Varie ...
File
... • Add solid base to acid (gently heat to speed up metal/carbonate to ensure all acid reaction reacts/neutralises and that the • Filter off excess solid base product is neutral • Heat filtrate solution until volume reduced by half • Leave solution to cool and allow remaining The percentage yield of c ...
... • Add solid base to acid (gently heat to speed up metal/carbonate to ensure all acid reaction reacts/neutralises and that the • Filter off excess solid base product is neutral • Heat filtrate solution until volume reduced by half • Leave solution to cool and allow remaining The percentage yield of c ...
Microbial-induced synthesis of nanoparticles of zinc phosphate and
... and 20 ml Paenibacillus mucilaginosus culture were added to above solution, respectively. The reaction solution was also allowed to stand under static conditions at room temperature for 24 hours. The precipitations were filtrated and washed three times with deionized water and ethanol, and then drie ...
... and 20 ml Paenibacillus mucilaginosus culture were added to above solution, respectively. The reaction solution was also allowed to stand under static conditions at room temperature for 24 hours. The precipitations were filtrated and washed three times with deionized water and ethanol, and then drie ...
as a PDF
... of irradiation pulses prevents this kind of redox process through electron transfer. It was shown that in the same mixed ion system the metal clusters obtained change with increasing dose rate from a bilayered core/shell structure to an alloyed structure or bimetallic solid solution.10 In the litera ...
... of irradiation pulses prevents this kind of redox process through electron transfer. It was shown that in the same mixed ion system the metal clusters obtained change with increasing dose rate from a bilayered core/shell structure to an alloyed structure or bimetallic solid solution.10 In the litera ...
H - Deans Community High School
... volume of (deionised) water in a beaker. • The solution is transferred to a standard flask. • The beaker is rinsed and the rinsings also poured into the standard flask. • The flask is made up to the mark adding the last few drops of water using a dropping pipette. • The flask is stoppered and invert ...
... volume of (deionised) water in a beaker. • The solution is transferred to a standard flask. • The beaker is rinsed and the rinsings also poured into the standard flask. • The flask is made up to the mark adding the last few drops of water using a dropping pipette. • The flask is stoppered and invert ...
Unit 14-Chemical Reactions
... • Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. • Symbols represent elements, formulas describe compounds, chemical equations describe a chemical reaction: 2 H2 + O2 → 2 H2O • Chemical reactions occur when bonds between the outermo ...
... • Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. • Symbols represent elements, formulas describe compounds, chemical equations describe a chemical reaction: 2 H2 + O2 → 2 H2O • Chemical reactions occur when bonds between the outermo ...
Week 7 - Acid-base, redox
... Classifying, Writing, and Balancing Redox Reactions We previously classified, wrote, and balanced precipitation, acid-base, and gas-forming reactions. Redox reactions have electron transfer, and that is what sets them apart from the other reaction types. With redox, one atom loses one or more electr ...
... Classifying, Writing, and Balancing Redox Reactions We previously classified, wrote, and balanced precipitation, acid-base, and gas-forming reactions. Redox reactions have electron transfer, and that is what sets them apart from the other reaction types. With redox, one atom loses one or more electr ...
ELECTRONIC MATERIALS Lecture 10
... These unoccupied energy levels enable carriers to gain energy when moving in an applied electric field. Electrons in a partially filled band therefore do contribute to the electrical conductivity of the material. Completely filled bands do contain plenty of electrons but do not contribute to the con ...
... These unoccupied energy levels enable carriers to gain energy when moving in an applied electric field. Electrons in a partially filled band therefore do contribute to the electrical conductivity of the material. Completely filled bands do contain plenty of electrons but do not contribute to the con ...
Congratulations! You have signed up for AP Chemistry for this year
... AP stands for “Advanced Placement” and AP Chemistry is the equivalent of a college chemistry class. There are some chemistry principles that are important that you remember from Pre-AP Chemistry, so I have compiled some practice notes and assignments that will equip you for the beginning of the year ...
... AP stands for “Advanced Placement” and AP Chemistry is the equivalent of a college chemistry class. There are some chemistry principles that are important that you remember from Pre-AP Chemistry, so I have compiled some practice notes and assignments that will equip you for the beginning of the year ...
Chemical Compounds Chemical Compounds
... heard the phrase “oil and water don’t mix.” Oil, such as that used in salad dressing, is composed of covalent compounds. Many covalent compounds do not dissolve well in water. Water molecules have a stronger attraction for one another than they have for the molecules of most other covalent compounds ...
... heard the phrase “oil and water don’t mix.” Oil, such as that used in salad dressing, is composed of covalent compounds. Many covalent compounds do not dissolve well in water. Water molecules have a stronger attraction for one another than they have for the molecules of most other covalent compounds ...
atomic mass
... • Positively charged center of an atom, containing nearly all of the atom’s mass • About 1/10,000 the size of the atom • Consists of two types of particles • Proton: Positively charged subatomic ...
... • Positively charged center of an atom, containing nearly all of the atom’s mass • About 1/10,000 the size of the atom • Consists of two types of particles • Proton: Positively charged subatomic ...
Chem 171 Review - Exam 1
... protons, electrons, neutrons - know the characteristics of these particles including relative masses and electrical charge atomic structure - where do the various subatomic particles reside? relative size and density of the atom and the nucleus atomic # vs. mass # - what are they, and what informati ...
... protons, electrons, neutrons - know the characteristics of these particles including relative masses and electrical charge atomic structure - where do the various subatomic particles reside? relative size and density of the atom and the nucleus atomic # vs. mass # - what are they, and what informati ...
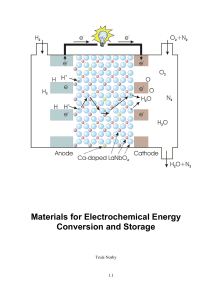
Materials for Electrochemical Energy Conversion and Storage
... Electrochemistry deals with the relation between chemical reactions and electrical phenomena – mainly potential and current of electrons. It is important for a large part of the metallurgical industry in which electrical energy is used to produce metals by electrolysis. Moreover, it is important for ...
... Electrochemistry deals with the relation between chemical reactions and electrical phenomena – mainly potential and current of electrons. It is important for a large part of the metallurgical industry in which electrical energy is used to produce metals by electrolysis. Moreover, it is important for ...
An Overview of Chemistry Lecture 3 Lecture 3
... Elements and compounds represent are substances, whose composition is fixed. • Every substance has a unique set of chemical and physical properties. • The properties of compounds are distinct from those of the elements from which they are made. Mixtures are composed of more than one substance. • The ...
... Elements and compounds represent are substances, whose composition is fixed. • Every substance has a unique set of chemical and physical properties. • The properties of compounds are distinct from those of the elements from which they are made. Mixtures are composed of more than one substance. • The ...