SAMPLE EXAMINATION IV Section I – Multiple Choice
... Questions 1-5. The set of lettered choices, a list of oxides, below refers to the numbered phrases immediately following it. Select the one lettered choice that is most closely associated with each numbered phrase. Each lettered choice can be used once, more than once or not at all. (A) SO2 (B) BaO2 ...
... Questions 1-5. The set of lettered choices, a list of oxides, below refers to the numbered phrases immediately following it. Select the one lettered choice that is most closely associated with each numbered phrase. Each lettered choice can be used once, more than once or not at all. (A) SO2 (B) BaO2 ...
The decomposition of hydrogen peroxide to form water and oxygen
... In the box below, draw the complete Lewis electron dot diagrams of a methanoic acid molecule and a water molecule in an orientation that allows a hydrogen bond to form between them. ...
... In the box below, draw the complete Lewis electron dot diagrams of a methanoic acid molecule and a water molecule in an orientation that allows a hydrogen bond to form between them. ...
Gas-Phase Reactions of Fe (CH2O)+ and Fe (CH2S)+ with Small
... Abstract: The gas-phase reactions of Fe(CH2O)+ and Fe(CH2S)+ with a series of aliphatic alkanes were studied by Fourier transform ion cyclotron resonance (FTICR) mass spectrometry. Like bare Fe+, C-C insertion, particularly terminal C-C insertion, is predominant for the reactions of Fe(CH2O)+, while ...
... Abstract: The gas-phase reactions of Fe(CH2O)+ and Fe(CH2S)+ with a series of aliphatic alkanes were studied by Fourier transform ion cyclotron resonance (FTICR) mass spectrometry. Like bare Fe+, C-C insertion, particularly terminal C-C insertion, is predominant for the reactions of Fe(CH2O)+, while ...
2 Atoms and Molecules
... Atoms of elements have no electrical charge and so must contain identical numbers of positive protons and negative electrons. However, because neutrons have no electrical charge, their numbers in an atom do not have to be the same as the numbers of protons or electrons. The number of protons in the ...
... Atoms of elements have no electrical charge and so must contain identical numbers of positive protons and negative electrons. However, because neutrons have no electrical charge, their numbers in an atom do not have to be the same as the numbers of protons or electrons. The number of protons in the ...
File
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
New Developments in Transmission Electron Microscopy for
... HRTEM.[4,5] Analysis of such tubular structures requires extensive development of electron microscopy, which has been covered very comprehensively in the book edited by Wang.[6] Conventional imaging and diffraction are the two most powerful methods in characterizing the phase structure and phase tra ...
... HRTEM.[4,5] Analysis of such tubular structures requires extensive development of electron microscopy, which has been covered very comprehensively in the book edited by Wang.[6] Conventional imaging and diffraction are the two most powerful methods in characterizing the phase structure and phase tra ...
Chapter 10 Chemical Bonding Theories
... Bonds form using shared electrons between overlapping orbitals on adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma and pi. ...
... Bonds form using shared electrons between overlapping orbitals on adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma and pi. ...
3.Redox
... 10. Suppose a 8.75 M aqueous CH3OH solution has a density of 0.789 g / mL. Calculate the mole fraction of CH3OH in the solution.. 11. A solution prepared by dissolving 6.0 g of NaCl in enough water to give 500 mL of solution has a density of 1.05 g / mL. Calculate (a) the Molarity of the solution, ( ...
... 10. Suppose a 8.75 M aqueous CH3OH solution has a density of 0.789 g / mL. Calculate the mole fraction of CH3OH in the solution.. 11. A solution prepared by dissolving 6.0 g of NaCl in enough water to give 500 mL of solution has a density of 1.05 g / mL. Calculate (a) the Molarity of the solution, ( ...
TDB-5: Standards and conventions for TDB publications
... The same chemical formula may refer to different chemical species and must often be specified more clearly in order to avoid ambiguities. For example, UF 4 occurs as a gas, a solid, and an aqueous complex. The distinction between the different phases is made by phase designators that immediately fol ...
... The same chemical formula may refer to different chemical species and must often be specified more clearly in order to avoid ambiguities. For example, UF 4 occurs as a gas, a solid, and an aqueous complex. The distinction between the different phases is made by phase designators that immediately fol ...
Muscle Lecture Test Questions – Set 3
... Heads (cross-bridges) detach during their contraction cycling, because: a. this is necessary to permit them to reattach to different actins along the thin filaments b. troponin is at an elevated level c. swiveling requires this to alter their angle of approach d. they do not have sufficient ATP e. o ...
... Heads (cross-bridges) detach during their contraction cycling, because: a. this is necessary to permit them to reattach to different actins along the thin filaments b. troponin is at an elevated level c. swiveling requires this to alter their angle of approach d. they do not have sufficient ATP e. o ...
chapter 15 acids and bases
... Step 1: Express the equilibrium concentrations of all species in terms of initial concentrations and a single unknown x, that represents the change in concentration. Let (−x) be the depletion in concentration (mol/L) of HF. From the stoichiometry of the reaction, it follows that the increase in conc ...
... Step 1: Express the equilibrium concentrations of all species in terms of initial concentrations and a single unknown x, that represents the change in concentration. Let (−x) be the depletion in concentration (mol/L) of HF. From the stoichiometry of the reaction, it follows that the increase in conc ...
ExamView - 1984 AP Chemistry Exam.tst
... between the vapor and liquid phases is positive. B) The slope of the curve representing equilibrium between the liquid and solid phases is negative. C) The slope of the curve representing equilibrium between the liquid and solid phases is positive. D) the temperature at the triple point is greater t ...
... between the vapor and liquid phases is positive. B) The slope of the curve representing equilibrium between the liquid and solid phases is negative. C) The slope of the curve representing equilibrium between the liquid and solid phases is positive. D) the temperature at the triple point is greater t ...
Brilliant Preparatory Section, Sitamarhi
... In using the term mole for ionic substances, we mean the number of formula units of the substance. For example, a mole of sodium carbonate, Na2CO3 is a quantity containing 6.023 x 1023 Na2CO3 units. But each formula unit of Na2CO3 contains 2 x 6.023 x 1023 Na+ ions and one CO32ions and 1 x 6.023 x 1 ...
... In using the term mole for ionic substances, we mean the number of formula units of the substance. For example, a mole of sodium carbonate, Na2CO3 is a quantity containing 6.023 x 1023 Na2CO3 units. But each formula unit of Na2CO3 contains 2 x 6.023 x 1023 Na+ ions and one CO32ions and 1 x 6.023 x 1 ...
Intermolecular forces and molecules
... Identify the ion-dipole attractions acting between the cation or anion and the water. In the sim, solvation is shown to be a dynamic process with H2O molecules moving about an ion. This is an improvement over static images showing the water always aligned in an optimal arrangement around an ion when ...
... Identify the ion-dipole attractions acting between the cation or anion and the water. In the sim, solvation is shown to be a dynamic process with H2O molecules moving about an ion. This is an improvement over static images showing the water always aligned in an optimal arrangement around an ion when ...
T-Shaped Molecular Building Units in the Porous Structure of Ag(4,4
... open frameworks with rectangular channels (c, bottom). In each case the assembly is accompanied by the inclusion of a guest molecule G which occupies the voids. ...
... open frameworks with rectangular channels (c, bottom). In each case the assembly is accompanied by the inclusion of a guest molecule G which occupies the voids. ...
Chapter 4 – Part 1
... protons, and neutrons present in an atom given its mass number. Know isotopes and how to write them Know most elements have at least two stable (non-radioactive) isotopes Know how to find the number of protons and neutrons in an isotope Know the 3 isotopes of hydrogen Know how to calculate the avera ...
... protons, and neutrons present in an atom given its mass number. Know isotopes and how to write them Know most elements have at least two stable (non-radioactive) isotopes Know how to find the number of protons and neutrons in an isotope Know the 3 isotopes of hydrogen Know how to calculate the avera ...
Insulator charging by contact with metals
... There are, however, divergences of opinion whether the electrons are transferred only to the surface or if they penetrate into the bulk of the insulator. The surface trap theory (Bauser et. al. (1970)) and the penetration theory (Davies (1967 a)) are indistinguishable in the sense that they both cor ...
... There are, however, divergences of opinion whether the electrons are transferred only to the surface or if they penetrate into the bulk of the insulator. The surface trap theory (Bauser et. al. (1970)) and the penetration theory (Davies (1967 a)) are indistinguishable in the sense that they both cor ...
2.6 M - Thierry Karsenti
... atoms in the cyclic structure is other than carbon. Heterocyclic componds may be aliphatic or aromatic 15. Isomers: These are different compounds that have the same molecular formula. Isomers are further subdivided into: (a) structural isomers, (b) geometrical isomers and (c) stereoisomers(optical i ...
... atoms in the cyclic structure is other than carbon. Heterocyclic componds may be aliphatic or aromatic 15. Isomers: These are different compounds that have the same molecular formula. Isomers are further subdivided into: (a) structural isomers, (b) geometrical isomers and (c) stereoisomers(optical i ...
Molecular diffusion at surfaces
... bonds and draws the charge from the metal atoms, leading to weakening of the metalmetal bonds. But carbon forms a covalent Fe 3d-C2s,2p interactions with iron there by gets more stabilized. The ionization potential input parameters for the C 2s and 2p orbitals are decreased by 1.5 eV from their atom ...
... bonds and draws the charge from the metal atoms, leading to weakening of the metalmetal bonds. But carbon forms a covalent Fe 3d-C2s,2p interactions with iron there by gets more stabilized. The ionization potential input parameters for the C 2s and 2p orbitals are decreased by 1.5 eV from their atom ...
sol-gel chemistry of transition metal oxides
... well known 3S , and detailed experimental data on the hydrolysis of cations can be found the ...
... well known 3S , and detailed experimental data on the hydrolysis of cations can be found the ...
Document
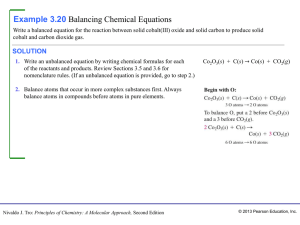
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...