WRITING CHEMICAL FORMULAE
... HCl particles which are involved. The water is just a carrier for the acid, so when we measure out a volume of the solution, we want to know how much acid it contains. To do this, we need to define the CONCENTRATION of the solution. This tells us how much solute there is in a fixed volume of the sol ...
... HCl particles which are involved. The water is just a carrier for the acid, so when we measure out a volume of the solution, we want to know how much acid it contains. To do this, we need to define the CONCENTRATION of the solution. This tells us how much solute there is in a fixed volume of the sol ...
The Properties of Ion-Water - Indiana University Bloomington
... very important role for anions. Contradictory results were reported by Stuart and Berne4 in a study on the hydration of the chloride ion, in which it was claimed that water, not chloride, polarizability is the critical property. Solvation properties of small 共n = 1 – 10兲 ion-water clusters using pol ...
... very important role for anions. Contradictory results were reported by Stuart and Berne4 in a study on the hydration of the chloride ion, in which it was claimed that water, not chloride, polarizability is the critical property. Solvation properties of small 共n = 1 – 10兲 ion-water clusters using pol ...
Tro Ch 3 Lecture PP - Highline Community College
... of atoms of each element in a compound it does not describe how many atoms, the order of attachment, or the shape the formulas for ionic compounds are empirical The empirical formula for the ionic compound fluorspar is CaCl2. This means that there is 1 Ca2+ ion for every 2 Cl− ions in the compound ...
... of atoms of each element in a compound it does not describe how many atoms, the order of attachment, or the shape the formulas for ionic compounds are empirical The empirical formula for the ionic compound fluorspar is CaCl2. This means that there is 1 Ca2+ ion for every 2 Cl− ions in the compound ...
Unit F325 - Equilibria, energetics and elements - High band
... Lattice enthalpies are negative values and use of the words ‘more’ and ‘less’ should not be used unless qualified for comparing negative numbers (–2 is actually ‘more’ than –4!). The candidate should have stated that the lattice enthalpy of MgO is less exothermic or less negative’. By using just ‘le ...
... Lattice enthalpies are negative values and use of the words ‘more’ and ‘less’ should not be used unless qualified for comparing negative numbers (–2 is actually ‘more’ than –4!). The candidate should have stated that the lattice enthalpy of MgO is less exothermic or less negative’. By using just ‘le ...
Homework1-4-Answers
... (b) neutron/proton ratio 1.00, 1.00, (11:9) 1.22, (4:3) 1.33, (13:9) 1.44 The neutron/proton ratio increases with increasing atomic number. 19.0 Identify each of the following elements: (a) a halogen whose anion contains 36 electrons, - Br(b) a radioactive noble gas with 86 protons, - Rn (c) a Group ...
... (b) neutron/proton ratio 1.00, 1.00, (11:9) 1.22, (4:3) 1.33, (13:9) 1.44 The neutron/proton ratio increases with increasing atomic number. 19.0 Identify each of the following elements: (a) a halogen whose anion contains 36 electrons, - Br(b) a radioactive noble gas with 86 protons, - Rn (c) a Group ...
- Career Point Kota
... (a) Zone refining : It is used to obtain metal of high purity. It is based on the principal that the impurities are more soluble in molten state than in the solid state. (b) Froth floatation process : It is used to concentrate sulphide ore. It is based on the fact that the mineral. Particles become ...
... (a) Zone refining : It is used to obtain metal of high purity. It is based on the principal that the impurities are more soluble in molten state than in the solid state. (b) Froth floatation process : It is used to concentrate sulphide ore. It is based on the fact that the mineral. Particles become ...
Document
... vacancy defect. This results the decrease in density of the substance. This defect develops when a substance is heated. # Interstitial defect- When some constituent particles occupy an interstitial site, the crystal is said to have interstitial defect. This defect increases the density of the substa ...
... vacancy defect. This results the decrease in density of the substance. This defect develops when a substance is heated. # Interstitial defect- When some constituent particles occupy an interstitial site, the crystal is said to have interstitial defect. This defect increases the density of the substa ...
9/10/10 1 Chemistry 121: Atomic and Molecular Chemistry
... A Molecule is a aggregate of at least two atoms in a definite arrangement held together by chemical forces (chemical bonds) Diatomic Molecules contain two atoms, Polyatomic Molecules more than 2 An ion is an atom or group of atoms with a net charge • Cation has a positive (+) charge Mg+2, Fe+3 Mona ...
... A Molecule is a aggregate of at least two atoms in a definite arrangement held together by chemical forces (chemical bonds) Diatomic Molecules contain two atoms, Polyatomic Molecules more than 2 An ion is an atom or group of atoms with a net charge • Cation has a positive (+) charge Mg+2, Fe+3 Mona ...
2E HARRY B. GRAY GEORGE S. HAMMONP.
... not developed in the text for reasons of space and which would normally be taken up in greater detail in later courses. The material in this volume has been adapted primarily from a portion of the lectures given by H. B. 6. and 6. S. fl. to the Chemistry 2 students at the California Institute of Tec ...
... not developed in the text for reasons of space and which would normally be taken up in greater detail in later courses. The material in this volume has been adapted primarily from a portion of the lectures given by H. B. 6. and 6. S. fl. to the Chemistry 2 students at the California Institute of Tec ...
NOBLE-GAS CHEMISTRY
... Sun et al. in 1996.30 In 1994, Thompson and Andrews synthesized novel adducts of noble gases to a metal center, NgBe(II)O, where Ng = Ar, Kr, and Xe.31 The case of XeBeO is rather straightforward: a coordinatively unsaturated Be(II) cation exposes its empty (sp) hybrid, and is ready to bind whatever ...
... Sun et al. in 1996.30 In 1994, Thompson and Andrews synthesized novel adducts of noble gases to a metal center, NgBe(II)O, where Ng = Ar, Kr, and Xe.31 The case of XeBeO is rather straightforward: a coordinatively unsaturated Be(II) cation exposes its empty (sp) hybrid, and is ready to bind whatever ...
c00kieee - Ritter Illustration
... Am has a half-life of 432.7 years. When a single crystal containing this highly radioactive material is isolated and analyzed by X-ray analysis, the intensity of the diffraction from the crystal has been observed to decrease with time, presumably as the crystal lattice is damaged. In the extreme, a ...
... Am has a half-life of 432.7 years. When a single crystal containing this highly radioactive material is isolated and analyzed by X-ray analysis, the intensity of the diffraction from the crystal has been observed to decrease with time, presumably as the crystal lattice is damaged. In the extreme, a ...
Dr. Spencer`s PPT
... – The sum of the oxidation numbers (total) must equal the charge of the molecule (including the neutral ...
... – The sum of the oxidation numbers (total) must equal the charge of the molecule (including the neutral ...
Atomic and Ionic Radii of Elements 1–96
... smaller than those of the neighboring Group 2 atoms. There is a possibility that by relying solely on the density we are, in fact, underestimating the Group 1 atom radii. The reason that the outermost electron density can be assumed a good measure of the size of an atom is because it relates[41] to ...
... smaller than those of the neighboring Group 2 atoms. There is a possibility that by relying solely on the density we are, in fact, underestimating the Group 1 atom radii. The reason that the outermost electron density can be assumed a good measure of the size of an atom is because it relates[41] to ...
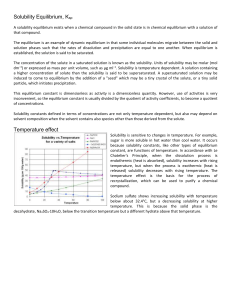
ksp - lozon.ca
... For some substances, formation of a solid or crystallization does not occur automatically whenever a solution is saturated. These substances have a tendency to form oversaturated solutions. For example, syrup and honey are oversaturated sugar solutions, containing other substances such as citric aci ...
... For some substances, formation of a solid or crystallization does not occur automatically whenever a solution is saturated. These substances have a tendency to form oversaturated solutions. For example, syrup and honey are oversaturated sugar solutions, containing other substances such as citric aci ...
ENTHALPY CHANGE DH
... distorted) and there is additional degree of covalent character within bonding. The smaller and more highly charged the metal ion the greater the deviation from the ionic model. ...
... distorted) and there is additional degree of covalent character within bonding. The smaller and more highly charged the metal ion the greater the deviation from the ionic model. ...
«Классы и номенклатура неорганических соединений»
... the methane molecule? A. *sp3 B. sp2 C. sp D.dsp2 E. sp3d2 17. For which molecules is not possess hydrogen bond? A. *CH4 B. NH3 C. HF D. H2O E. C2H5OH 18. What is the maximal valence of fluorine? A. *1 B. 7 C. 2 D. 5 E. 3 19. What is the structure of water molecule? A. *angle B. line C. square D. cu ...
... the methane molecule? A. *sp3 B. sp2 C. sp D.dsp2 E. sp3d2 17. For which molecules is not possess hydrogen bond? A. *CH4 B. NH3 C. HF D. H2O E. C2H5OH 18. What is the maximal valence of fluorine? A. *1 B. 7 C. 2 D. 5 E. 3 19. What is the structure of water molecule? A. *angle B. line C. square D. cu ...
Hubbard-U is necessary on ligand atom for predicting
... ELDAU [ n( r )] ELDA [ n( r )] EHub [{ nmI }] EDC [{ n I }] n(r) is the electronic density nmI the atomic orbital occupations for the atom I experiencing the “Hubbard” term The last term in the above equation is then subtracted in order to avoid double counting of the interactions con ...
... ELDAU [ n( r )] ELDA [ n( r )] EHub [{ nmI }] EDC [{ n I }] n(r) is the electronic density nmI the atomic orbital occupations for the atom I experiencing the “Hubbard” term The last term in the above equation is then subtracted in order to avoid double counting of the interactions con ...
silbchp4
... Must see a change in oxidation numbers from reactants to products LEO says GER (Loss of Electrons is Oxidation; Gain of Electrons is Reduction) ...
... Must see a change in oxidation numbers from reactants to products LEO says GER (Loss of Electrons is Oxidation; Gain of Electrons is Reduction) ...
study material(2014-15) class xii-chemistry
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
chemistry-resource
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
View
... 1,3,5-triazines containing coordinating groups other than azaheterocycles have been only scarcely explored, for example some interesting coordination complexes being provided by the triphosphine 2,4,6-tris(diphenylphosphino)-1,3,5-triazine.14 Besides the phosphino groups, which coordinate metals thr ...
... 1,3,5-triazines containing coordinating groups other than azaheterocycles have been only scarcely explored, for example some interesting coordination complexes being provided by the triphosphine 2,4,6-tris(diphenylphosphino)-1,3,5-triazine.14 Besides the phosphino groups, which coordinate metals thr ...
Interaction of the Adenine-Thymine Watson
... group IIb (Zn2+, Cd2+, Hg2+) divalent cations with the adenineadenine reverse-Hoogsteen (AA rH) base pair. Also, a complex of the adenine-thymine Watson-Crick (AT WC) base pair with hydrated Mg2+ ion has been studied. We did not consider interactions of the other cations with the AT WC pair since th ...
... group IIb (Zn2+, Cd2+, Hg2+) divalent cations with the adenineadenine reverse-Hoogsteen (AA rH) base pair. Also, a complex of the adenine-thymine Watson-Crick (AT WC) base pair with hydrated Mg2+ ion has been studied. We did not consider interactions of the other cations with the AT WC pair since th ...
Chemistry
... substances[3] that are constituted of atoms or the subatomic particles: protons, electrons and neutrons.[4] Atoms combine to produce molecules or crystals. Chemistry is sometimes called "the central science" because it connects the other natural sciences such as astronomy, physics, material science, ...
... substances[3] that are constituted of atoms or the subatomic particles: protons, electrons and neutrons.[4] Atoms combine to produce molecules or crystals. Chemistry is sometimes called "the central science" because it connects the other natural sciences such as astronomy, physics, material science, ...
Lecture 25 Notes
... A hydrogen ion (H+) does not exist by itself in an aqueous solution • In water, H+ combines with a polar H2O molecule to form a hydrated hydrogen ion (H3O+) called a hydronium ion ...
... A hydrogen ion (H+) does not exist by itself in an aqueous solution • In water, H+ combines with a polar H2O molecule to form a hydrated hydrogen ion (H3O+) called a hydronium ion ...