CHM 423 Coordination Chemistry
... From his experiment, a complex containing chloride(s) which gave precipitate on reacting with AgNO3 solution was said to be an electrolyte while non-electrolyte gave no precipitate. The precipitated chloride satisfed only primary valency i.e. it was outside the coordination sphere while un-precipita ...
... From his experiment, a complex containing chloride(s) which gave precipitate on reacting with AgNO3 solution was said to be an electrolyte while non-electrolyte gave no precipitate. The precipitated chloride satisfed only primary valency i.e. it was outside the coordination sphere while un-precipita ...
"Positron-impact ionization, positronium formation, and electronic excitation cross sections for diatomic molecules" Phys. Rev. A 72 (2005), 062713. J. P. Marler and C.M. Surko (PDF)
... Positrons from a 22Na radioactive source and neon moderator are trapped and cooled in a three-stage buffer-gas PenningMalmberg trap in a 0.15 T magnetic field. The positrons cool to the temperature of the buffer gas and surrounding electrodes 共i.e., 300 K ⬅ 25 meV兲 in ⬃0.1 s. The process of positron ...
... Positrons from a 22Na radioactive source and neon moderator are trapped and cooled in a three-stage buffer-gas PenningMalmberg trap in a 0.15 T magnetic field. The positrons cool to the temperature of the buffer gas and surrounding electrodes 共i.e., 300 K ⬅ 25 meV兲 in ⬃0.1 s. The process of positron ...
Effect of phospholipid and (phospho)lipase - Annales UMCS
... phospholipid dispersed systems zeta potential (surface charge), particle size, temperature, pH and ionic strength are important parameters for their characteristics and stability. The pH and ionic strength changes induce those in the electrical charge of phospholipid layer or liposome surface. This ...
... phospholipid dispersed systems zeta potential (surface charge), particle size, temperature, pH and ionic strength are important parameters for their characteristics and stability. The pH and ionic strength changes induce those in the electrical charge of phospholipid layer or liposome surface. This ...
Homework 5-7 answers
... 5. If equal masses of O2(g) and HBr(g) are in separate containers of equal volume and temperature, which one of these statements is true? A) The pressure in the O2 container is greater than that in the HBr container. B) There are more HBr molecules than O2 molecules. C) The average velocity of the O ...
... 5. If equal masses of O2(g) and HBr(g) are in separate containers of equal volume and temperature, which one of these statements is true? A) The pressure in the O2 container is greater than that in the HBr container. B) There are more HBr molecules than O2 molecules. C) The average velocity of the O ...
Electronic structure of molecular van der Waals complexes with
... ~as in H2O/benzene! and induced dipole-induced dipole interactions ~in all complexes!, another phenomenon playing a role in the stability of most of the complexes is the presence of a small but significant charge transfer from benzene to the small molecules. The charge transfer, as obtained from a M ...
... ~as in H2O/benzene! and induced dipole-induced dipole interactions ~in all complexes!, another phenomenon playing a role in the stability of most of the complexes is the presence of a small but significant charge transfer from benzene to the small molecules. The charge transfer, as obtained from a M ...
Chapter 5: Gases - HCC Learning Web
... 5. If equal masses of O2(g) and HBr(g) are in separate containers of equal volume and temperature, which one of these statements is true? A) The pressure in the O2 container is greater than that in the HBr container. B) There are more HBr molecules than O2 molecules. C) The average velocity of the O ...
... 5. If equal masses of O2(g) and HBr(g) are in separate containers of equal volume and temperature, which one of these statements is true? A) The pressure in the O2 container is greater than that in the HBr container. B) There are more HBr molecules than O2 molecules. C) The average velocity of the O ...
Chapter 22 - 2012 Book Archive
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
Homework 5-8 answers
... C) The average velocity of the O2 molecules is less than that of the HBr molecules. D) The average kinetic energy of HBr molecules is greater than that of O2 molecules. E) The pressures of both gases are the same. Ans: A Page 85 Chapter 5: Gases 6. Deviations from the ideal gas law are greater at A ...
... C) The average velocity of the O2 molecules is less than that of the HBr molecules. D) The average kinetic energy of HBr molecules is greater than that of O2 molecules. E) The pressures of both gases are the same. Ans: A Page 85 Chapter 5: Gases 6. Deviations from the ideal gas law are greater at A ...
CHAPTER 4 - Myschoolpages.com
... The Ions complete the circuit by carrying electrical charge between the electrodes Chapt. 4.2 ...
... The Ions complete the circuit by carrying electrical charge between the electrodes Chapt. 4.2 ...
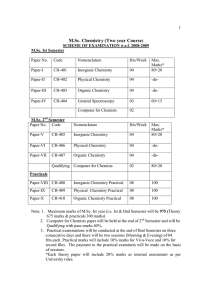
M.Sc. Chemistry (Two year Course)
... Section-A Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensiona ...
... Section-A Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensiona ...
Chapter 1: Chemistry: The Study of Change
... (a) a halogen whose anion contains 36 electrons, - Br(b) a radioactive noble gas with 86 protons, - Rn (c) a Group 6A element whose anion contains 36 electrons, - Se-2 (d) an alkali metal cation that contains 36 electrons, - Rb+ (e) a Group 4A cation that contains 80 electrons. – Pb+2 20.0 Some comp ...
... (a) a halogen whose anion contains 36 electrons, - Br(b) a radioactive noble gas with 86 protons, - Rn (c) a Group 6A element whose anion contains 36 electrons, - Se-2 (d) an alkali metal cation that contains 36 electrons, - Rb+ (e) a Group 4A cation that contains 80 electrons. – Pb+2 20.0 Some comp ...
Document
... molecule of O2 is 0, since the molecule is produced by species of the same (not different) element(s)] ...
... molecule of O2 is 0, since the molecule is produced by species of the same (not different) element(s)] ...
Document
... molecule of O2 is 0, since the molecule is produced by species of the same (not different) element(s)] ...
... molecule of O2 is 0, since the molecule is produced by species of the same (not different) element(s)] ...
5 organic chemistry: functional groups
... Methanol, or methyl alcohol, is also known as wood alcohol because it was originally made by heating wood until a liquid distilled. Methanol is highly toxic, and many people have become blind or have died from drinking it. Ethanol, or ethyl alcohol, is the alcohol associated with “alcoholic” beverag ...
... Methanol, or methyl alcohol, is also known as wood alcohol because it was originally made by heating wood until a liquid distilled. Methanol is highly toxic, and many people have become blind or have died from drinking it. Ethanol, or ethyl alcohol, is the alcohol associated with “alcoholic” beverag ...
Chemistry Unit 1
... is also important to realize that hydroxides which react with both acids and bases are described as amphoteric substances. For example, aluminium hydroxide, Al(OH)3, reacts with both acids and bases to form salt and water. So, Al(OH)3, is amphoteric in nature. What is the common characteristic of ac ...
... is also important to realize that hydroxides which react with both acids and bases are described as amphoteric substances. For example, aluminium hydroxide, Al(OH)3, reacts with both acids and bases to form salt and water. So, Al(OH)3, is amphoteric in nature. What is the common characteristic of ac ...
Has the Periodic Table Been Successfully Axiomatized?
... claim regarding the status of the periodic law. The following section Hettema and Kuiper’s article consists of a brief and generally accurate account of the early historical development of the periodic table. The only important omission would seem to be the author’s failure to mention the experiment ...
... claim regarding the status of the periodic law. The following section Hettema and Kuiper’s article consists of a brief and generally accurate account of the early historical development of the periodic table. The only important omission would seem to be the author’s failure to mention the experiment ...
1970 - Warren County Schools
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
C:\SUBJECTS\SUBJECTS\Chemistry
... In the reaction Fe + Cu2+ Fe2+ + Cu, iron displaces copper ions to form copper. This is due to the fact that A. iron is in the metallic form while dthe copper is in the ionic form B. the atomic weight of copper is greater than that of ion C. copper metal has more electrons than ion metal D. iron is ...
... In the reaction Fe + Cu2+ Fe2+ + Cu, iron displaces copper ions to form copper. This is due to the fact that A. iron is in the metallic form while dthe copper is in the ionic form B. the atomic weight of copper is greater than that of ion C. copper metal has more electrons than ion metal D. iron is ...
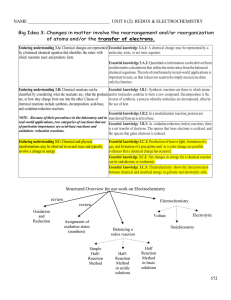
Unit 7: Reduction, Oxidation and Electrochemistry
... Examples: CO2, CO, SO3, SO2, CO32−, SO42− and H2O all have an oxidation number of −2 for oxygen. An exception occurs in peroxides (compound containing O22−) where the oxygen has an oxidation number of −1. 4. Hydrogen in Binary Compounds and Polyatomic Ions has an Oxidation Number of +1. Examples: H2 ...
... Examples: CO2, CO, SO3, SO2, CO32−, SO42− and H2O all have an oxidation number of −2 for oxygen. An exception occurs in peroxides (compound containing O22−) where the oxygen has an oxidation number of −1. 4. Hydrogen in Binary Compounds and Polyatomic Ions has an Oxidation Number of +1. Examples: H2 ...
Synthesis and Characterisation of N
... In the periodic table, groups are usually referred to by Arabic or Roman ...
... In the periodic table, groups are usually referred to by Arabic or Roman ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
The polydentate ligands include polyaminopolycarbonic acids, such
... 7. The nomenclature of complex compounds The name of the complex compounds are composed as follows: Name the ligands first, in alphabetical order, then the central atom or ion. Name of the complex is written in one word. Neutral ligands called without changes; in the names of negatively charged liga ...
... 7. The nomenclature of complex compounds The name of the complex compounds are composed as follows: Name the ligands first, in alphabetical order, then the central atom or ion. Name of the complex is written in one word. Neutral ligands called without changes; in the names of negatively charged liga ...
chemistry - Textbooks Online
... strands of philosophy from Greece, China, Egypt and Arabia mixed in. The main aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), and the search for the philosopher’s stone, which would turn base metals into ...
... strands of philosophy from Greece, China, Egypt and Arabia mixed in. The main aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), and the search for the philosopher’s stone, which would turn base metals into ...
Disproportionation of Gold(II)
... be linear, two-coordinate complexes, whereas the latter are typically square planar, four-coordinate complexes. The chemistry of the intermediate formal oxidation state, divalent gold (i.e., AuII), has come under increasing scrutiny in terms of its chemical applications as well as the novelty of thi ...
... be linear, two-coordinate complexes, whereas the latter are typically square planar, four-coordinate complexes. The chemistry of the intermediate formal oxidation state, divalent gold (i.e., AuII), has come under increasing scrutiny in terms of its chemical applications as well as the novelty of thi ...
Unit- 5.pmd
... Another important factor featuring adsorption is the heat of adsorption. During adsorption, there is always a decrease in residual forces of the surface, i.e., there is decrease in surface energy which appears as heat. Adsorption, therefore, is invariably an exothermic process. In other words, ∆H of ...
... Another important factor featuring adsorption is the heat of adsorption. During adsorption, there is always a decrease in residual forces of the surface, i.e., there is decrease in surface energy which appears as heat. Adsorption, therefore, is invariably an exothermic process. In other words, ∆H of ...