PowerPoint Lectures - Northwest ISD Moodle
... Solutions • Solutions are defined as homogeneous mixtures of two or more pure substances. • Aqueous solution – solution in which water is the dissolving medium • The solvent is present in greatest abundance. • All other substances are solutes; they are dissolved in the solvent. – Example: NaCl diss ...
... Solutions • Solutions are defined as homogeneous mixtures of two or more pure substances. • Aqueous solution – solution in which water is the dissolving medium • The solvent is present in greatest abundance. • All other substances are solutes; they are dissolved in the solvent. – Example: NaCl diss ...
Solid State Physics
... filled with electrons, while all levels above EF are empty. • Electrons are free to move into “empty” states of conduction band with only a small electric field E, leading to high electrical conductivity! • At T > 0, electrons have a probability to be thermally “excited” from below the Fermi energy ...
... filled with electrons, while all levels above EF are empty. • Electrons are free to move into “empty” states of conduction band with only a small electric field E, leading to high electrical conductivity! • At T > 0, electrons have a probability to be thermally “excited” from below the Fermi energy ...
Chemistry Final - Practice Test I
... Filtration An electrical process that can separate water into hydrogen and oxygen. Electrolysis Boiling a chemical and then condensing its vapors. Distillation ...
... Filtration An electrical process that can separate water into hydrogen and oxygen. Electrolysis Boiling a chemical and then condensing its vapors. Distillation ...
Final Exam - Dawson College
... Smallest atomic radius in Group 6A Largest atomic radius in Period 6 Condensed ground-state electron configuration is [Ne] 3s23p2 The period 4 member whose (2-) ion is isoelectronic with Kr A transition metal ion with a charge of 1+ having 5 unpaired “4d” electrons The element with the highest first ...
... Smallest atomic radius in Group 6A Largest atomic radius in Period 6 Condensed ground-state electron configuration is [Ne] 3s23p2 The period 4 member whose (2-) ion is isoelectronic with Kr A transition metal ion with a charge of 1+ having 5 unpaired “4d” electrons The element with the highest first ...
Photosynthesis Stores Energy in Organic Compounds
... which moves to a higher energy level electron passes to the electron-accepting molecule. • After this, the electron moves through 4 steps ...
... which moves to a higher energy level electron passes to the electron-accepting molecule. • After this, the electron moves through 4 steps ...
Stoichiometry 1 amu = 1.6606 x 10-24 g The amu mass of an atom
... Stoichiometry 1 amu = 1.6606 x 10-24 g The amu mass of an atom of carbon-12 is 12 amu 1 mole = count multiplier = 6.022 x 1023 items subscript to the right of an element symbol = atom count multiplier = the number of atoms of the element in a chemical formula number before chemical formula in a chem ...
... Stoichiometry 1 amu = 1.6606 x 10-24 g The amu mass of an atom of carbon-12 is 12 amu 1 mole = count multiplier = 6.022 x 1023 items subscript to the right of an element symbol = atom count multiplier = the number of atoms of the element in a chemical formula number before chemical formula in a chem ...
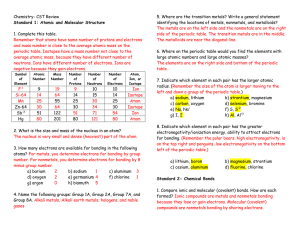
Chemistry- CST Review
... 8. Indicate which element in each pair has the greater electronegativity/ionization energy, ability to attract electrons for bonding. (Remember the polar bears, high electronegativity, is on the top right and penguins, low electronegativity on the bottom left of the periodic table.) a) lithium, boro ...
... 8. Indicate which element in each pair has the greater electronegativity/ionization energy, ability to attract electrons for bonding. (Remember the polar bears, high electronegativity, is on the top right and penguins, low electronegativity on the bottom left of the periodic table.) a) lithium, boro ...
Chemistry Module 1- Basic Revision Notes 1.1a Atomic Structure 1.1
... 1.1.3 Elements (H, He, Li, Be,…..) are the basic building blocks of all matter, and cannot be broken down into simpler parts by chemical means. 1.1.4 There is a clear relationship between an elements electronic structure and its position in the periodic table. P E r i o d ...
... 1.1.3 Elements (H, He, Li, Be,…..) are the basic building blocks of all matter, and cannot be broken down into simpler parts by chemical means. 1.1.4 There is a clear relationship between an elements electronic structure and its position in the periodic table. P E r i o d ...
Photosynthesis Stores Energy in Organic Compounds
... which moves to a higher energy level electron passes to the electron-accepting molecule. • After this, the electron moves through 4 steps ...
... which moves to a higher energy level electron passes to the electron-accepting molecule. • After this, the electron moves through 4 steps ...
Aqueous chemistry is a very important component to laboratory
... They are not doing much of anything – but when you drop those tablets in water, then away go the gas bubbles. The active ingredients in Alka-Seltzer are sodium bicarbonate, NaHCO3, and citric acid, H3C6H5O7. When water is added to an Alka-Seltzer tablet, the bica ...
... They are not doing much of anything – but when you drop those tablets in water, then away go the gas bubbles. The active ingredients in Alka-Seltzer are sodium bicarbonate, NaHCO3, and citric acid, H3C6H5O7. When water is added to an Alka-Seltzer tablet, the bica ...
mass Spectrometry (mS)
... NOTE: Because the positive ion formed has an unpaired electron it is sometimes shown with a dot indicating that it is a free radical, e.g. CH3+• The positive ions are then ACCELERATED by an electric field and focused into a fine beam by passing through a series of slits with increasing negative pote ...
... NOTE: Because the positive ion formed has an unpaired electron it is sometimes shown with a dot indicating that it is a free radical, e.g. CH3+• The positive ions are then ACCELERATED by an electric field and focused into a fine beam by passing through a series of slits with increasing negative pote ...
Electrostatics Intro (6/16)
... on an electrically neutral piece of paper. This is because A. electrons are less massive than atomic nuclei. B. the electric force between charged particles decreases with increasing distance. C. an atomic nucleus occupies only a small part of the volume of an atom. D. a typical atom has many electr ...
... on an electrically neutral piece of paper. This is because A. electrons are less massive than atomic nuclei. B. the electric force between charged particles decreases with increasing distance. C. an atomic nucleus occupies only a small part of the volume of an atom. D. a typical atom has many electr ...
Oxidation and Reduction - UCLA Chemistry and Biochemistry
... bonds between a carbon and atoms that are less electronegative than carbon (often hydrogen). ...
... bonds between a carbon and atoms that are less electronegative than carbon (often hydrogen). ...
Grade 11 Chemistry Exam Review
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
CHEMONE Directions: Select the letter of the best
... 40. 1.000 atm of dry nitrogen, placed in a container having a pinhole opening in its side, leaks from the container 3.55 times faster than does 1.000 atm of an unknown gas placed in the same apparatus. Which of these species could be the unknown gas? a. NH3 b. C4H10 c. SF6 d. UF6 e. Rn 41. Which of ...
... 40. 1.000 atm of dry nitrogen, placed in a container having a pinhole opening in its side, leaks from the container 3.55 times faster than does 1.000 atm of an unknown gas placed in the same apparatus. Which of these species could be the unknown gas? a. NH3 b. C4H10 c. SF6 d. UF6 e. Rn 41. Which of ...
Chapter 3
... 33. An anion is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable atom. C. a group of stable atoms. D. an atom or group of atoms with a net positive charge. 34. An cation is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable a ...
... 33. An anion is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable atom. C. a group of stable atoms. D. an atom or group of atoms with a net positive charge. 34. An cation is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable a ...
Chapter 4: Chemical Reactions Elements can be characterized as
... For a binary compound AX, the oxidation number is the number of electrons gained or lost by an atom of the element when it forms the compound. It is sometimes referred to as the oxidation state. Oxidation numbers (Table 4-10) are used to track electron transfer in oxidation-reduction (redox) reactio ...
... For a binary compound AX, the oxidation number is the number of electrons gained or lost by an atom of the element when it forms the compound. It is sometimes referred to as the oxidation state. Oxidation numbers (Table 4-10) are used to track electron transfer in oxidation-reduction (redox) reactio ...
Instructor`s Notes Atomic Tiles: Play Your Way from Atoms to
... 5a. Students know reactant atoms and molecules interact to form products with different chemical properties. 5b. Students know the idea of atoms explains the conservation of matter: In chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays th ...
... 5a. Students know reactant atoms and molecules interact to form products with different chemical properties. 5b. Students know the idea of atoms explains the conservation of matter: In chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays th ...
Ideas To Implementation
... weak and could not produce a loud sound without being amplified Achieved amplification by using vacuum tubes (thermionic devices), which were very fragile, large, expensive and required high power consumption The invention of the transistor solved these significant shortcomings: o Contained 3 se ...
... weak and could not produce a loud sound without being amplified Achieved amplification by using vacuum tubes (thermionic devices), which were very fragile, large, expensive and required high power consumption The invention of the transistor solved these significant shortcomings: o Contained 3 se ...
AP Chemistry Jeopardy
... States of Matter $200 Under what conditions would a substance sublimate at room conditions (25 °C and 1 atm)? A) the critical temperature must be below the triple point B) the slope of the fusion line should be negative C) the triple point must be at a pressure greater than 1 atm and 25°C D) the so ...
... States of Matter $200 Under what conditions would a substance sublimate at room conditions (25 °C and 1 atm)? A) the critical temperature must be below the triple point B) the slope of the fusion line should be negative C) the triple point must be at a pressure greater than 1 atm and 25°C D) the so ...
1 - Groupfusion.net
... 40. An ionic bond forms between what types of elements? A metal and a nonmetal An ionic bond is the attraction between positively charged metal cations and negatively charged anions. In an ionic bond, electrons are transferred from the metal (cation) to the nonmetal (anion). What is the structure of ...
... 40. An ionic bond forms between what types of elements? A metal and a nonmetal An ionic bond is the attraction between positively charged metal cations and negatively charged anions. In an ionic bond, electrons are transferred from the metal (cation) to the nonmetal (anion). What is the structure of ...
chemistry in the 8th grade
... configuration. This would be 2 electrons in the first shell and 8 electrons in any shell after the first one. Atoms can undergo chemical reactions by losing, gaining, or sharing electrons to achieve this stable configuration. If atoms have 3 or fewer outer electrons, they can lose these electrons to ...
... configuration. This would be 2 electrons in the first shell and 8 electrons in any shell after the first one. Atoms can undergo chemical reactions by losing, gaining, or sharing electrons to achieve this stable configuration. If atoms have 3 or fewer outer electrons, they can lose these electrons to ...
Matter
... One factor that makes suspensions different from solutions is the size of the particles that make up the solution. The particles in a solution are tiny ions or molecules. The particles in suspension are much larger. Because they are larger, the particles in a suspension do not dissolve. Be sure to a ...
... One factor that makes suspensions different from solutions is the size of the particles that make up the solution. The particles in a solution are tiny ions or molecules. The particles in suspension are much larger. Because they are larger, the particles in a suspension do not dissolve. Be sure to a ...
Part One: Molecular Geometry and Directional Bonding A
... Now that we can predict molecular geometry, we can predict polarity in a molecule. ...
... Now that we can predict molecular geometry, we can predict polarity in a molecule. ...