Grade 10 NSC Chemistry Curriculum
... covalent molecules, names and formulae of covalent compounds. • Ionic bonding: transfer of electrons in the formation of ionic bonding, cations and anions, electron diagrams of simple ionic compounds. Ionic structure as illustrated by sodium chloride • Revise the writing of names when given the form ...
... covalent molecules, names and formulae of covalent compounds. • Ionic bonding: transfer of electrons in the formation of ionic bonding, cations and anions, electron diagrams of simple ionic compounds. Ionic structure as illustrated by sodium chloride • Revise the writing of names when given the form ...
Unit 7 Chap. 7 Chemical Formulas and Compounds
... 7. THE ALGEBRAIC SUM OF THE OXIDATION NUMBERS OF ALL ATOMS IN A NEUTRAL COMPOUND IS ZERO. 8. THE ALGEBRAIC SUM OF THE OXIDATION NUMBERS OF ALL ATOMS IN A POLYATOMIC ION IS EQUAL TO THE CHARGE OF THE ION. ...
... 7. THE ALGEBRAIC SUM OF THE OXIDATION NUMBERS OF ALL ATOMS IN A NEUTRAL COMPOUND IS ZERO. 8. THE ALGEBRAIC SUM OF THE OXIDATION NUMBERS OF ALL ATOMS IN A POLYATOMIC ION IS EQUAL TO THE CHARGE OF THE ION. ...
Rxn Pred students
... by the passage of current under sufficient external electrical potential. The devices in which electrolysis reactions occur are called electrolytic cells. ...
... by the passage of current under sufficient external electrical potential. The devices in which electrolysis reactions occur are called electrolytic cells. ...
High School Chemistry
... the emission of high-energy radiation results from nuclear changes and that matter can be converted to energy in nuclear reactions. Objective 1: Identify how the quantum energy changes in the atom in terms of light emission. a. Identify the relationship between energy and wavelength of light. b. Cor ...
... the emission of high-energy radiation results from nuclear changes and that matter can be converted to energy in nuclear reactions. Objective 1: Identify how the quantum energy changes in the atom in terms of light emission. a. Identify the relationship between energy and wavelength of light. b. Cor ...
- Catalyst
... E) none of the above 14. What is the mass % of H in ammonium phosphate ((NH4)3PO3? A) 2.3% B) 6.0% C) 9.1% D) 17% E) none of the above 15. Naturally occurring rubidium has an atomic mass of 85.5amu. It is composed of two isotopes, rubidium–85 (84.9amu) and rubidium–87 (86.9amu). From this informatio ...
... E) none of the above 14. What is the mass % of H in ammonium phosphate ((NH4)3PO3? A) 2.3% B) 6.0% C) 9.1% D) 17% E) none of the above 15. Naturally occurring rubidium has an atomic mass of 85.5amu. It is composed of two isotopes, rubidium–85 (84.9amu) and rubidium–87 (86.9amu). From this informatio ...
∙ ∙B x
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
∙ ∙B x
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
PHYSICAL SETTING CHEMISTRY
... questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, whic ...
... questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, whic ...
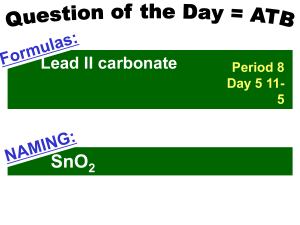
Naming Compounds
... o - rather large difference in electronegativity of the bonding elements – made of monatomic ions, polyatomic ions, and/or both – monatomic ions: consist of a single atom {e.g. Na+ or F-} - polyatomic ions: consist of more than one atom {e.g. OH-, NH4+ } ...
... o - rather large difference in electronegativity of the bonding elements – made of monatomic ions, polyatomic ions, and/or both – monatomic ions: consist of a single atom {e.g. Na+ or F-} - polyatomic ions: consist of more than one atom {e.g. OH-, NH4+ } ...
2007 - Thompson Rivers University
... on your performance. Top performers are eligible for prizes. The contest consists of 25 multiple choice questions. You have 60 minutes to complete the test. All questions are of equal value, there is no particular order to the questions and there is no penalty for incorrect answers. Please answer on ...
... on your performance. Top performers are eligible for prizes. The contest consists of 25 multiple choice questions. You have 60 minutes to complete the test. All questions are of equal value, there is no particular order to the questions and there is no penalty for incorrect answers. Please answer on ...
summer assignment File - District 196 e
... Complete “interactive practice exercises” for each section as needed. (“Interactive practice exercises” are also on Mr. Allan’s website.) Complete “interactive review exercises” for each chapter. Study/repeat “interactive review exercises” until you are at least 85% (preferably 90%) accurate. (Y ...
... Complete “interactive practice exercises” for each section as needed. (“Interactive practice exercises” are also on Mr. Allan’s website.) Complete “interactive review exercises” for each chapter. Study/repeat “interactive review exercises” until you are at least 85% (preferably 90%) accurate. (Y ...
Midterm Review - Closter Public Schools
... liquids, they _____________________________. In gases they ________________________. Matter is said to be ______________ when it is has only one type of particle. Matter is said to be ______________when it has more than one type of particle. A ______________ is a pure substance that contains only a ...
... liquids, they _____________________________. In gases they ________________________. Matter is said to be ______________ when it is has only one type of particle. Matter is said to be ______________when it has more than one type of particle. A ______________ is a pure substance that contains only a ...
Review for Chapter 6: Thermochemistry
... hybrid orbitals are employed. The inclusion of the d orbital enables “expanded octets” to occur. For octahedral molecules in which six pairs of electrons are arranged around a central atom, sp3d2 hybrid orbitals are used. 10. Sigma bonds are covalent bonds formed by orbitals overlapping end-to-end, ...
... hybrid orbitals are employed. The inclusion of the d orbital enables “expanded octets” to occur. For octahedral molecules in which six pairs of electrons are arranged around a central atom, sp3d2 hybrid orbitals are used. 10. Sigma bonds are covalent bonds formed by orbitals overlapping end-to-end, ...
Molecular Geometry
... 1. Add up the total # of valence electrons for all the atoms. Account for charge: If the species has a negative (–) charge: add one valence electron for each negative charge; for a positively charged (+) species, subtract one electron for each positive charge. 2. Draw the molecular skeleton and conn ...
... 1. Add up the total # of valence electrons for all the atoms. Account for charge: If the species has a negative (–) charge: add one valence electron for each negative charge; for a positively charged (+) species, subtract one electron for each positive charge. 2. Draw the molecular skeleton and conn ...
ppt
... distributions in molecules but cannot explain the properties of some molecules. O O O 2: VB theory O: Is2 2s2 2p4 sp2 hybridized O, one sp2 from each forms s-bond and the other two are occupied with the lone pairs. The un-hybridized p on each forms the p-bond Indicates that in O2 molecule, all elect ...
... distributions in molecules but cannot explain the properties of some molecules. O O O 2: VB theory O: Is2 2s2 2p4 sp2 hybridized O, one sp2 from each forms s-bond and the other two are occupied with the lone pairs. The un-hybridized p on each forms the p-bond Indicates that in O2 molecule, all elect ...
Gr 10 Review sheet chemistry
... 1. Change of________________ 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...
... 1. Change of________________ 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...
Chemistry Content Standards
... physical processes. a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. b. Collect data and calculate the amount of heat given off or taken in by chemical or physical processes. c. Analyzing (both conceptually and quantitatively) flow of energy during change of st ...
... physical processes. a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. b. Collect data and calculate the amount of heat given off or taken in by chemical or physical processes. c. Analyzing (both conceptually and quantitatively) flow of energy during change of st ...
Exam 1 Review Sheet
... 1. Read (and/or re-read) the entire assignment. 2. Review your notes from lecture and from the text. 3. In addition to the important concepts listed below, make sure you understand all of the facts and concepts Chang has summarized at the end of each chapter along with the key words he has listed. 4 ...
... 1. Read (and/or re-read) the entire assignment. 2. Review your notes from lecture and from the text. 3. In addition to the important concepts listed below, make sure you understand all of the facts and concepts Chang has summarized at the end of each chapter along with the key words he has listed. 4 ...
Objective 3 Stations Student Sheet
... 1. How is the periodic table organized? 2. What family of elements has valence electrons at two energy levels? 3. What are the elements called that are between metals and nonmetals? 4. Which family of nonmetals has seven valence electrons? 5. What are some properties of noble gases? 6. What is anoth ...
... 1. How is the periodic table organized? 2. What family of elements has valence electrons at two energy levels? 3. What are the elements called that are between metals and nonmetals? 4. Which family of nonmetals has seven valence electrons? 5. What are some properties of noble gases? 6. What is anoth ...
How do you tell if a molecule is paramagnetic or diamagnetic
... oxygen for example. According to VB theory, each oxygen atom has 3 sp2 hybrid orbitals, there is one sigma-bond formed by the overlap of a pair of sp2 hybrid orbitals from each atom, one pi-bond formed by the overlap of atomic p-orbitals and each oxygen atom has 2 non-bonding pairs in 2 sp2 hybrid o ...
... oxygen for example. According to VB theory, each oxygen atom has 3 sp2 hybrid orbitals, there is one sigma-bond formed by the overlap of a pair of sp2 hybrid orbitals from each atom, one pi-bond formed by the overlap of atomic p-orbitals and each oxygen atom has 2 non-bonding pairs in 2 sp2 hybrid o ...
1 - 嘉義大學
... 20. If the concentration of the product were to double, what would happen to the equilibrium constant? (A) It would double its value. (B) It would become half its current value. (C) It would quadruple its value. (D) It would not change its value. 21. What statement about equilibrium is true? (A) Whe ...
... 20. If the concentration of the product were to double, what would happen to the equilibrium constant? (A) It would double its value. (B) It would become half its current value. (C) It would quadruple its value. (D) It would not change its value. 21. What statement about equilibrium is true? (A) Whe ...
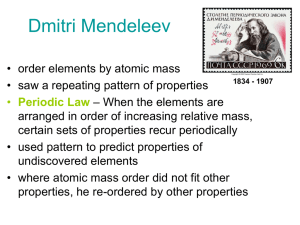
Dmitri Mendeleev
... Every element wants to be like the noble gases wants 0 or 8 valence electrons valence e- (outer electron shell used for bonding) ...
... Every element wants to be like the noble gases wants 0 or 8 valence electrons valence e- (outer electron shell used for bonding) ...
Chemistry Definitions
... isotopic masses and physical properties 5. Isoelectronic: Same number of electrons 6. Isotonic: Same number of neutrons 7. Isotopic: Same number of protons 8. Principal Quantum Number: describes the main energy level of an electron and the size of an atomic orbital 9. Subshells: subdivision of each ...
... isotopic masses and physical properties 5. Isoelectronic: Same number of electrons 6. Isotonic: Same number of neutrons 7. Isotopic: Same number of protons 8. Principal Quantum Number: describes the main energy level of an electron and the size of an atomic orbital 9. Subshells: subdivision of each ...