SCATTERING OF ELECTRONS BY DIATOMIC MOLECULES IN
... Ridley[lOJ with allowance for exchange (these curves are given also in[ 5 J). As seen from the figures, the approximation of two small-radius wells give fair results at medium incidentelectron energies. The main advantage of the model, however, lies in the fact that it can be readily generalized to ...
... Ridley[lOJ with allowance for exchange (these curves are given also in[ 5 J). As seen from the figures, the approximation of two small-radius wells give fair results at medium incidentelectron energies. The main advantage of the model, however, lies in the fact that it can be readily generalized to ...
Atomic Theory (2
... 1.) What are the 5 characteristics of ideal gases? 2.) What is the volume of one mole of any gas at STP? 3.) At what temperature would 2.10 moles of N2 gas have a pressure of 1.25 atm and in a 25.0 L tank? 4.) What volume is occupied by 5.03 g of O2 at 28°C and a pressure of 0.998atm? 5.) What is th ...
... 1.) What are the 5 characteristics of ideal gases? 2.) What is the volume of one mole of any gas at STP? 3.) At what temperature would 2.10 moles of N2 gas have a pressure of 1.25 atm and in a 25.0 L tank? 4.) What volume is occupied by 5.03 g of O2 at 28°C and a pressure of 0.998atm? 5.) What is th ...
AP Semestar Exam REVIEW
... a. takes a proton from an Arrhenius acid. b. is reduced in a chemical reaction. c. gains electrons in a chemical reaction. d. loses electrons in a chemical reaction. e. gives a proton to an Arrhenius base. ____ 26. Assign oxidation numbers to each atom in calcium perchlorate, Ca(ClO4)2. a. Ca = 0; C ...
... a. takes a proton from an Arrhenius acid. b. is reduced in a chemical reaction. c. gains electrons in a chemical reaction. d. loses electrons in a chemical reaction. e. gives a proton to an Arrhenius base. ____ 26. Assign oxidation numbers to each atom in calcium perchlorate, Ca(ClO4)2. a. Ca = 0; C ...
Chapter 2 - Chemistry
... 2.) Some metallic elements have more than one cation. These elements have common cations with a charge equal to the group number minus 2, in addition to having a cation with a charge equal to the group number. 3.) Most transition elements form more than one cation. Most of these elements have one io ...
... 2.) Some metallic elements have more than one cation. These elements have common cations with a charge equal to the group number minus 2, in addition to having a cation with a charge equal to the group number. 3.) Most transition elements form more than one cation. Most of these elements have one io ...
Chemical Reactions Chemistry - is the study of matter, its properties
... Most common acids are known because of the combination of hydrogen with one of the elements from the halogen family. They are considered acids because they dissociate or separate into ions easily in water. Molecular Compounds Molecular compounds are formed when two non-metals combine. Molecular comp ...
... Most common acids are known because of the combination of hydrogen with one of the elements from the halogen family. They are considered acids because they dissociate or separate into ions easily in water. Molecular Compounds Molecular compounds are formed when two non-metals combine. Molecular comp ...
Dr. Ali Ebneshahidi © 2016 Ebneshahidi
... atoms tend to change also – atoms that have either lost or gained electrons are called ions. Atoms that have lost electrons (as a result, now contain more p+ than e-) are called cations which carry positive charges, while atoms that have gained excessive electrons (as a result, now contain more etha ...
... atoms tend to change also – atoms that have either lost or gained electrons are called ions. Atoms that have lost electrons (as a result, now contain more p+ than e-) are called cations which carry positive charges, while atoms that have gained excessive electrons (as a result, now contain more etha ...
X273/13/02
... your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one correct answer to each question. 8 Any rough working should be done on the question paper or the rough working sheet, not on ...
... your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one correct answer to each question. 8 Any rough working should be done on the question paper or the rough working sheet, not on ...
Final Exam review semester 1
... The reaction in Figure 7-1 shows the formation of ammonia from nitrogen and hydrogen in the Haber process. What will be the effect on the equilibrium if the temperature is increased and some of the ammonia is removed from the system? ____ ____ ...
... The reaction in Figure 7-1 shows the formation of ammonia from nitrogen and hydrogen in the Haber process. What will be the effect on the equilibrium if the temperature is increased and some of the ammonia is removed from the system? ____ ____ ...
Microsoft Word
... All acetates are soluble except for Be(CH3COO)2 All phosphates are insoluble except for those of Group I elements and NH4+. All carbonates are insoluble except for those of Group I elements and NH4+. All hydroxides are insoluble except for those of NH4+, Group I, Sr(OH)2, and Ba(OH)2; Ca(OH)2 is sli ...
... All acetates are soluble except for Be(CH3COO)2 All phosphates are insoluble except for those of Group I elements and NH4+. All carbonates are insoluble except for those of Group I elements and NH4+. All hydroxides are insoluble except for those of NH4+, Group I, Sr(OH)2, and Ba(OH)2; Ca(OH)2 is sli ...
end of year review
... A. Atoms are mostly composed of empty space. B. All matter is composed of tiny, discrete particles called atoms. C. Electrons orbit the nucleus of an atom at distinct energy levels. D. Atoms are composed of positively and negatively charged particles. ...
... A. Atoms are mostly composed of empty space. B. All matter is composed of tiny, discrete particles called atoms. C. Electrons orbit the nucleus of an atom at distinct energy levels. D. Atoms are composed of positively and negatively charged particles. ...
Foreign molecules and ions in beryl obtained by infrared and visible
... both symmetric stretching, and with deformation vibrations at 1627 cm-1 and 1632 cm-1 for type II. In range of symmetric stretching there is broad vibrational band which can be explained by presence of water molecules type II near alkali ions. Overtones and combinations of these fundamental vibratio ...
... both symmetric stretching, and with deformation vibrations at 1627 cm-1 and 1632 cm-1 for type II. In range of symmetric stretching there is broad vibrational band which can be explained by presence of water molecules type II near alkali ions. Overtones and combinations of these fundamental vibratio ...
Chapter 4 - Colby College Wiki
... • Write the equations for the half-reactions. – Balance all atoms except H and O (balance H and O also if they undergo redox) – Add e- based on oxidation state changes – Balance oxygen atoms using H2O – Balance hydrogen atoms using H+ • Equalize the number of electrons. • Add the half reactions. • I ...
... • Write the equations for the half-reactions. – Balance all atoms except H and O (balance H and O also if they undergo redox) – Add e- based on oxidation state changes – Balance oxygen atoms using H2O – Balance hydrogen atoms using H+ • Equalize the number of electrons. • Add the half reactions. • I ...
Atomic Structure - s3.amazonaws.com
... Dalton’s Atomic Theory (Between 1766-1844) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. Chemical reactions when atoms are separated, joined, or rearranged. Atoms of one element are never changed into another ele ...
... Dalton’s Atomic Theory (Between 1766-1844) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. Chemical reactions when atoms are separated, joined, or rearranged. Atoms of one element are never changed into another ele ...
name chemistry final review
... first find the molar mass of the compound and “dump” the unit you don’t want anymore!) *For extra names help – try to name the following compounds in the margin. a. 100g of BaCl2 0.5 moles of BaCl2 b. 35g of (NH4)3PO4 0.23 moles of (NH4)3PO4 c. 750g of MgS 13 moles of MgS d. 0.821g of BF3 0.0121 mol ...
... first find the molar mass of the compound and “dump” the unit you don’t want anymore!) *For extra names help – try to name the following compounds in the margin. a. 100g of BaCl2 0.5 moles of BaCl2 b. 35g of (NH4)3PO4 0.23 moles of (NH4)3PO4 c. 750g of MgS 13 moles of MgS d. 0.821g of BF3 0.0121 mol ...
Atomic Number… - Taylor County Schools
... • Each element contains a unique positive charge in their nucleus. • The number of protons in the nucleus of an atom identifies the element and is known as the ...
... • Each element contains a unique positive charge in their nucleus. • The number of protons in the nucleus of an atom identifies the element and is known as the ...
Chapter 4: Solution Chemistry and the Hydrosphere
... aqueous solution: a solution where water is the dissolving medium (the solvent) • For example, when table salt (NaCl) is dissolved in water, it results in an aqueous solution of sodium chloride, NaCl(aq), with Na+ and Cl- ions dissolved in water. • Note: The physical state aqueous,(aq), indicates an ...
... aqueous solution: a solution where water is the dissolving medium (the solvent) • For example, when table salt (NaCl) is dissolved in water, it results in an aqueous solution of sodium chloride, NaCl(aq), with Na+ and Cl- ions dissolved in water. • Note: The physical state aqueous,(aq), indicates an ...
Final Exam - W09
... A civil engineer wants to reduce odors at a wastewater treatment plant by adding hydrogen peroxide to the sewage. The hydrogen peroxide is delivered as 50% by mass solution, but for maintenance and safety issues, the H2O2 is diluted to a 3% by mass solution. If the engineer needs 20.0 gallons of the ...
... A civil engineer wants to reduce odors at a wastewater treatment plant by adding hydrogen peroxide to the sewage. The hydrogen peroxide is delivered as 50% by mass solution, but for maintenance and safety issues, the H2O2 is diluted to a 3% by mass solution. If the engineer needs 20.0 gallons of the ...
Give reasons for the following: (i) Bond enthalpy of F2
... Helium mixed with oxygen under pressure is given to sea-divers for respiration. Air is not given to sea-divers because nitrogen present in air being soluble in blood will give a painful sensation called bends by bubbling out blood on moving from high pressure(in deep sea) to the atmospheric pressure ...
... Helium mixed with oxygen under pressure is given to sea-divers for respiration. Air is not given to sea-divers because nitrogen present in air being soluble in blood will give a painful sensation called bends by bubbling out blood on moving from high pressure(in deep sea) to the atmospheric pressure ...
Covalent Bonding - whitburnscience
... All discrete covalent molecules have low melting and boiling points and tend to be liquids and gases at room temperature. Between all molecules in the liquid or solid state weak forces called van der waals’ forces exist these forces become larger as the size of the molecule increases, it is these fo ...
... All discrete covalent molecules have low melting and boiling points and tend to be liquids and gases at room temperature. Between all molecules in the liquid or solid state weak forces called van der waals’ forces exist these forces become larger as the size of the molecule increases, it is these fo ...
Review Sheet Exam 2 3.4-4.7
... 7. True/False When diluting a solution the number of moles changes. 8. What are the 2 components and a solution? 9. How many moles K2Cr2O7 is present in 250mL of 0.025M solution? ...
... 7. True/False When diluting a solution the number of moles changes. 8. What are the 2 components and a solution? 9. How many moles K2Cr2O7 is present in 250mL of 0.025M solution? ...
Chemistry Mid-Term Review Sheet
... 51. How did Mendeleev arrange his periodic table? 52. How is the modern periodic table arranged? 53. What determines an element’s chemical properties? 54. Define cation and anion. 55. The radius of a cation is ___________ than its neutral atom. 56. The radius of an anion is ___________than its neutr ...
... 51. How did Mendeleev arrange his periodic table? 52. How is the modern periodic table arranged? 53. What determines an element’s chemical properties? 54. Define cation and anion. 55. The radius of a cation is ___________ than its neutral atom. 56. The radius of an anion is ___________than its neutr ...
Chapter 13
... • Spectator Ions – Ions that do not take part in a chemical reaction and are found in solution both before and after the reaction ...
... • Spectator Ions – Ions that do not take part in a chemical reaction and are found in solution both before and after the reaction ...
Types of Materials
... the +1 or +2 valency state. The +2 oxidation state is the more stable of the two. The metal is resistant to corrosion and has low electrical resistance and good thermal conductivity. It is easily worked and can be joined by soldering, brazing and welding. It is used in water pipes, electrical wires ...
... the +1 or +2 valency state. The +2 oxidation state is the more stable of the two. The metal is resistant to corrosion and has low electrical resistance and good thermal conductivity. It is easily worked and can be joined by soldering, brazing and welding. It is used in water pipes, electrical wires ...
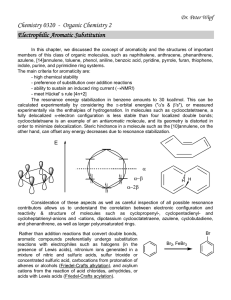
In this chapter, alkanes, alkenes, alkynes
... The resonance energy stabilization in benzene amounts to 30 kcal/mol. This can be calculated experimentally by considering the -orbital energies ("'s & 's"), or measured experimentally via the enthalpies of hydrogenation. In molecules such as cyclooctatetraene, a fully delocalized -electron conf ...
... The resonance energy stabilization in benzene amounts to 30 kcal/mol. This can be calculated experimentally by considering the -orbital energies ("'s & 's"), or measured experimentally via the enthalpies of hydrogenation. In molecules such as cyclooctatetraene, a fully delocalized -electron conf ...