Oxidation-Reduction (Redox) Reactions
... First determine oxidation numbers of each species in the reaction and then identify the oxidation or reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain electrons (be reduced). B. Reducing Agent- the substance that caus ...
... First determine oxidation numbers of each species in the reaction and then identify the oxidation or reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain electrons (be reduced). B. Reducing Agent- the substance that caus ...
I 14-7 ION CHEMISTRY
... into molecular ions with a range of internal energy states (Section 13-3). In some collisions the minimum energy required to cause ionization, the ionization energy IE, is transferred to the molecule. This situation leads to formation of the ion AB+· in its ground state, with zero internal energy. I ...
... into molecular ions with a range of internal energy states (Section 13-3). In some collisions the minimum energy required to cause ionization, the ionization energy IE, is transferred to the molecule. This situation leads to formation of the ion AB+· in its ground state, with zero internal energy. I ...
sample paper chemistry clas xi set 3
... (c) A mixture if a dil. NaOH and aluminuim piece is used to open holes. (d) Carbon shows catenation but silicon does not. (e) Tin (II) is a reducing agent but Pb(II) is not. ...
... (c) A mixture if a dil. NaOH and aluminuim piece is used to open holes. (d) Carbon shows catenation but silicon does not. (e) Tin (II) is a reducing agent but Pb(II) is not. ...
Chemistry 515 Name: L. S. Curtin Soc. Sec. #: February 8, 1999
... c) Isotopes of a given element have very different chemical reactivities because they have the same number of protons in the nucleus. d) Alkaline earth elements generally form +1 ions when combining to form compounds. e) None of the above are correct statements. Protons and neutrons reside in the nu ...
... c) Isotopes of a given element have very different chemical reactivities because they have the same number of protons in the nucleus. d) Alkaline earth elements generally form +1 ions when combining to form compounds. e) None of the above are correct statements. Protons and neutrons reside in the nu ...
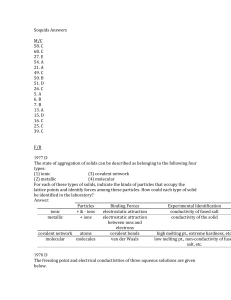
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... The freezing point of an aqueous solution is lower than the freezing point of water. A higher molality of a solution lowers the freezing point more and an equimolar amount of the two solids gives a larger molal solution from the calcium chloride as illustrated by the above equations. (b) Water is mo ...
... The freezing point of an aqueous solution is lower than the freezing point of water. A higher molality of a solution lowers the freezing point more and an equimolar amount of the two solids gives a larger molal solution from the calcium chloride as illustrated by the above equations. (b) Water is mo ...
4.6 Oxidation-Reduction (Redox) Reactions Oxidation Reduction
... First determine oxidation numbers of each species in the reaction and then identify the oxidation and reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain those electrons (be reduced). B. Reducing Agent- the substance th ...
... First determine oxidation numbers of each species in the reaction and then identify the oxidation and reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain those electrons (be reduced). B. Reducing Agent- the substance th ...
Ch. 8 Notes (Chemical Reactions) Teacher Relearn
... Step 1-- use your ion sheet and find the ions and their charges. Step 2-- “Cross the charges” if they don’t balance out. Step 3-- Use parentheses around polyatomic ion “chunks”. Practice Problems: Write the formula for each ionic compound. ...
... Step 1-- use your ion sheet and find the ions and their charges. Step 2-- “Cross the charges” if they don’t balance out. Step 3-- Use parentheses around polyatomic ion “chunks”. Practice Problems: Write the formula for each ionic compound. ...
Atoms and Molecules - Gulfport School District
... • All organisms are composed of energy and matter • All matter is composed of atoms • Elements are pure substances that contain only one type of atom and cannot be separated into simpler substances. • Atoms have a central core called the nucleus, which is composed of protons and neutrons that determ ...
... • All organisms are composed of energy and matter • All matter is composed of atoms • Elements are pure substances that contain only one type of atom and cannot be separated into simpler substances. • Atoms have a central core called the nucleus, which is composed of protons and neutrons that determ ...
Unit 1
... 3. To use Lewis dot symbols for atoms and ions as a model that shows valence electrons. 4. To use the “octet” rule to predict and describe the configuration of main group monatomic ions. 5. To know that cations are smaller than their atoms and anions are larger, and why. 6. To know that conductivity ...
... 3. To use Lewis dot symbols for atoms and ions as a model that shows valence electrons. 4. To use the “octet” rule to predict and describe the configuration of main group monatomic ions. 5. To know that cations are smaller than their atoms and anions are larger, and why. 6. To know that conductivity ...
Oxidation-Reduction Reactions
... Oxidation-reduction reactions, or redox reactions, are technically defined as any chemical reaction in which the oxidation number of the participating atom, ion, or molecule of a chemical compound changes. Some common redox reactions include fire, rusting of metals, browning of fruit, and photosynth ...
... Oxidation-reduction reactions, or redox reactions, are technically defined as any chemical reaction in which the oxidation number of the participating atom, ion, or molecule of a chemical compound changes. Some common redox reactions include fire, rusting of metals, browning of fruit, and photosynth ...
Describe properties of particles and thermochemical - Mr
... There are three major trends in the periodic table that you will need to explain. They are atomic and ionic radii, ionisation energy, and electronegativity. When attempting to explain these trends you will need to consider the relative size of the electrostatic attraction between the protons in the ...
... There are three major trends in the periodic table that you will need to explain. They are atomic and ionic radii, ionisation energy, and electronegativity. When attempting to explain these trends you will need to consider the relative size of the electrostatic attraction between the protons in the ...
Formation of Solutions
... When sodium hydroxide dissolved in water, it becomes warmer. Opposite this, if Ammonium Nitrate is dissolved in water, the solution becomes colder. ...
... When sodium hydroxide dissolved in water, it becomes warmer. Opposite this, if Ammonium Nitrate is dissolved in water, the solution becomes colder. ...
CHEMISTRY 1
... 1. Electron deficient molecules: Molecules where the central atom does not have an octet. Usually a group IIIA atom ...
... 1. Electron deficient molecules: Molecules where the central atom does not have an octet. Usually a group IIIA atom ...
periodic table - Mesa Community College
... major difference between CO (a compound called carbon monoxide) and Co (the element cobalt). The symbols of the elements can be represented in tabular form called the Periodic Table (Figure 1). This consists of vertical rows called families or groups and horizontal rows ...
... major difference between CO (a compound called carbon monoxide) and Co (the element cobalt). The symbols of the elements can be represented in tabular form called the Periodic Table (Figure 1). This consists of vertical rows called families or groups and horizontal rows ...
TRANSPORT OF IONS IN SOLUTION
... F accelerates cations to the negatively charged electrode and anions in the opposite direction. Through this motion, ions experience a frictional force in the opposite direction. Taking the expression derived by Stoke relating friction and the viscosity of the solvent (), it follows: Ffric 6rs, ...
... F accelerates cations to the negatively charged electrode and anions in the opposite direction. Through this motion, ions experience a frictional force in the opposite direction. Taking the expression derived by Stoke relating friction and the viscosity of the solvent (), it follows: Ffric 6rs, ...
Identify the following properties as either - Teach-n-Learn-Chem
... 34. Why do metals generally have lower ionizations energies than nonmetals? Metals have loosely held valence electrons. Metals want to lose electrons to have a stable octet. It is easier for a metal to lose 1,2, 3, or 4 electrons than gain that number. Metals have low electronegativities and are not ...
... 34. Why do metals generally have lower ionizations energies than nonmetals? Metals have loosely held valence electrons. Metals want to lose electrons to have a stable octet. It is easier for a metal to lose 1,2, 3, or 4 electrons than gain that number. Metals have low electronegativities and are not ...
eBook AQA GCSE Chemistry Unit C2 Part 1
... Ammonia gas exists as molecules. A molecule is a particle made up of two or more atoms chemically bonded together. In ammonia, each molecule consists of one atom of nitrogen joined to three atoms of hydrogen. The atoms are held together by covalent bonds. A covalent bond is a shared pair of electron ...
... Ammonia gas exists as molecules. A molecule is a particle made up of two or more atoms chemically bonded together. In ammonia, each molecule consists of one atom of nitrogen joined to three atoms of hydrogen. The atoms are held together by covalent bonds. A covalent bond is a shared pair of electron ...
1. What are micelles? Give two examples of micellar systems. Sol. A
... Anode reaction: 2Fe => 2Fe2+ + 4eCathode reaction: O2 + 2H2O + 4e- => 4OHThere are obviously different anodic and cathodic reactions for different alloys exposed to various environments. These half cell reactions are thought to occur (at least initially) at microscopic anodes and cathodes covering a ...
... Anode reaction: 2Fe => 2Fe2+ + 4eCathode reaction: O2 + 2H2O + 4e- => 4OHThere are obviously different anodic and cathodic reactions for different alloys exposed to various environments. These half cell reactions are thought to occur (at least initially) at microscopic anodes and cathodes covering a ...
On the Modeling of the Production and Drift of Carriers in
... simulated using these values. However, it is incorrectly estimated that a large amount of electrons drift all the way to the anode (Fig. 2). Since the effective attachment rate in liquids is a function of electric field [15], a constant, zero-field attachment rate does not reproduce the fact that at ...
... simulated using these values. However, it is incorrectly estimated that a large amount of electrons drift all the way to the anode (Fig. 2). Since the effective attachment rate in liquids is a function of electric field [15], a constant, zero-field attachment rate does not reproduce the fact that at ...
Chapter 4 Study Guide-Atomic Structure Define the following terms
... Atomic Mass Unit (amu)-unit of mass of a proton or neutron (1 amu each) Atomic number-number of protons, periodic table Dalton’s Atomic Theory-first theory to relate chemical changes to events at the atomic level Electron-negatively charged subatomic particle, lives outside of the nucleus Group-vert ...
... Atomic Mass Unit (amu)-unit of mass of a proton or neutron (1 amu each) Atomic number-number of protons, periodic table Dalton’s Atomic Theory-first theory to relate chemical changes to events at the atomic level Electron-negatively charged subatomic particle, lives outside of the nucleus Group-vert ...
Chemistry Unit Summaries - Oak Park Unified School District
... forming an expanded octet. When the total number of valence electrons is an odd number, then it will be necessary to place seven electrons around the atom with the odd number of valence electrons; usually nitrogen. When there are multiple valid Lewis structures for a molecule or ion, we can determin ...
... forming an expanded octet. When the total number of valence electrons is an odd number, then it will be necessary to place seven electrons around the atom with the odd number of valence electrons; usually nitrogen. When there are multiple valid Lewis structures for a molecule or ion, we can determin ...
SCSD Physical Science 9th - Shenandoah Community Schools
... It can be transformed from one state to another (I,D,M) • Understand energy transformations (I,D,M) o Energy can be transferred by: Collisions in chemical and nuclear reactions (I,D,M) Light waves and other radiations (I,D,M) o As transfers occur, the matter becomes steadily less ordered (I,D,M) • U ...
... It can be transformed from one state to another (I,D,M) • Understand energy transformations (I,D,M) o Energy can be transferred by: Collisions in chemical and nuclear reactions (I,D,M) Light waves and other radiations (I,D,M) o As transfers occur, the matter becomes steadily less ordered (I,D,M) • U ...
Chemistry II Aqueous Reactions and Solution Chemistry Chapter 4
... are substances that ionize in aqueous solutions to form hydrogen ions, increasing the concentration of hydrogen ions in solution. Because hydrogen ions are just a proton, acids are known as proton ...
... are substances that ionize in aqueous solutions to form hydrogen ions, increasing the concentration of hydrogen ions in solution. Because hydrogen ions are just a proton, acids are known as proton ...
class 2.pptx
... matter is composed of atoms. ✿ All atoms of a given element have identical chemical properties. ✿ Atoms of different elements have different properties. ✿ Atoms combine in whole number ratios to form compounds. ✿ Atoms are neither created nor destroyed in chemical reactions. ...
... matter is composed of atoms. ✿ All atoms of a given element have identical chemical properties. ✿ Atoms of different elements have different properties. ✿ Atoms combine in whole number ratios to form compounds. ✿ Atoms are neither created nor destroyed in chemical reactions. ...