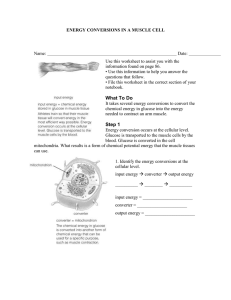
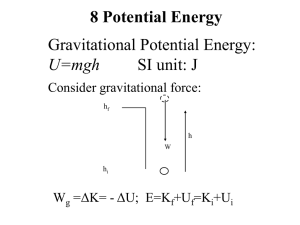
1-37 The First Law of Thermodynamics
... internal energy of the system increases by an amount that is equal to the work done on the system. This increase in the internal energy can be an increase in the internal potential energy, an increase in the internal kinetic energy, or both. An increase in the internal kinetic energy would manifest ...
... internal energy of the system increases by an amount that is equal to the work done on the system. This increase in the internal energy can be an increase in the internal potential energy, an increase in the internal kinetic energy, or both. An increase in the internal kinetic energy would manifest ...
Slide 1
... G. Potential Energy- the energy an object has because of its position or shape. It has energy because work has been already done to it. ...
... G. Potential Energy- the energy an object has because of its position or shape. It has energy because work has been already done to it. ...
Learning Target #1: Distinguish between kinetic and potential
... 6. When a 5 N book is removed from a 10-m shelf and placed on a table 1-m off the ground, what is the change in gravitational potential energy? ...
... 6. When a 5 N book is removed from a 10-m shelf and placed on a table 1-m off the ground, what is the change in gravitational potential energy? ...
Chapter 13: Work and Energy - South Kingstown High School
... energy ALWAYS REMAINS THE SAME We know where it goes. Can’t always use the new energy form, but it is counted ...
... energy ALWAYS REMAINS THE SAME We know where it goes. Can’t always use the new energy form, but it is counted ...
Mass Balance for Open System
... - We are sure that temperature will rise, since the heat will stimulate the atomic energy in the metals. - The question are; ...
... - We are sure that temperature will rise, since the heat will stimulate the atomic energy in the metals. - The question are; ...
Physical Science MidTerm Exam Study Guide
... 9. Although the Statue of Liberty is made of copper (originally an orange-brown color), it is green because the copper has interacted with substances in the air to form new substances with different properties. This is an example of what kind of change? 10. Flammability, solubility, and reactivity a ...
... 9. Although the Statue of Liberty is made of copper (originally an orange-brown color), it is green because the copper has interacted with substances in the air to form new substances with different properties. This is an example of what kind of change? 10. Flammability, solubility, and reactivity a ...
Internal energy is a characteristic of a given state – it is the same no
... Calculate entropy change in phase change or calculate entropy change with small temperature change (approximate with average temperature) Small temp change or small energy transfer between two objects at different temps. Look at total entropy! The equations show that the entropy for a closed system ...
... Calculate entropy change in phase change or calculate entropy change with small temperature change (approximate with average temperature) Small temp change or small energy transfer between two objects at different temps. Look at total entropy! The equations show that the entropy for a closed system ...
Chemistry 221 - Oregon State chemistry
... Name some common forms of energy that you encounter in your daily life and how you sense them. ...
... Name some common forms of energy that you encounter in your daily life and how you sense them. ...
Physical Science Final Exam Study Guide Part 2
... 49. Energy that travels through space in the form of waves 50. Energy stored in chemical bonds 51. The ability to do work 52. Energy associated with motion and position 53. Energy associated with electrical charge 54. Energy that depends on an object’s height (Ch 16) 55. Heat flows spontaneously fro ...
... 49. Energy that travels through space in the form of waves 50. Energy stored in chemical bonds 51. The ability to do work 52. Energy associated with motion and position 53. Energy associated with electrical charge 54. Energy that depends on an object’s height (Ch 16) 55. Heat flows spontaneously fro ...
Document
... If 4 N of frictional force is acting between the floor and block, what distance will the block go? W = DKE = f d d = DKE / f = 4.8 J / 4 N = 1.2 m ...
... If 4 N of frictional force is acting between the floor and block, what distance will the block go? W = DKE = f d d = DKE / f = 4.8 J / 4 N = 1.2 m ...
Energy Vocabulary, Grade 4
... Name _______________________ Energy Vocabulary, Grade 4 Directions: Prepare the definitions for the following science words. Add words as needed. ...
... Name _______________________ Energy Vocabulary, Grade 4 Directions: Prepare the definitions for the following science words. Add words as needed. ...
Jeopardy
... Which of Newton’s Laws states: Acceleration is produced when a force acts on a mass. The greater the mass, the greater the amount of force that is needed (to accelerate the object). ...
... Which of Newton’s Laws states: Acceleration is produced when a force acts on a mass. The greater the mass, the greater the amount of force that is needed (to accelerate the object). ...
CHAPTER TWO The First Law and Other Basic Concepts
... regarded as positive. Heat Q and work W always refer to the system, and the modern sign convention makes the numerical values of both quantities positive for transfer into the system from the surroundings. The corresponding quantities taken with reference to the surroundings, Qsur. and Wsur. have th ...
... regarded as positive. Heat Q and work W always refer to the system, and the modern sign convention makes the numerical values of both quantities positive for transfer into the system from the surroundings. The corresponding quantities taken with reference to the surroundings, Qsur. and Wsur. have th ...