TOPIC: Energy AIM: What is energy?
... 1. PE and KE both increase. 2. PE and KE both decrease. 3. PE decreases and KE increases. 4. PE increases and KE decreases. ...
... 1. PE and KE both increase. 2. PE and KE both decrease. 3. PE decreases and KE increases. 4. PE increases and KE decreases. ...
CHAPTER 6 - Thermochemistry
... w- work done by the system(w is negative) on its surroundings or done on the system(w is positive) by its surroundings. Ex. A piston full of gases absorbs 70 kJ of heat, causing the gases in the piston to expand and do 50 kJ of work on the surroundings. What is the change in internal energy of the g ...
... w- work done by the system(w is negative) on its surroundings or done on the system(w is positive) by its surroundings. Ex. A piston full of gases absorbs 70 kJ of heat, causing the gases in the piston to expand and do 50 kJ of work on the surroundings. What is the change in internal energy of the g ...
Conservative forces and potential energy
... Consider the more complicated situation in which the force on the particle is given by F(x) = x − 4qx 3 This is a conservative force and the its potential energy is U(x) = − 21 x 2 + qx 4 (see Fig. 7–10). From the force, we can calculate the motion using Newton’s second law. The program that you wil ...
... Consider the more complicated situation in which the force on the particle is given by F(x) = x − 4qx 3 This is a conservative force and the its potential energy is U(x) = − 21 x 2 + qx 4 (see Fig. 7–10). From the force, we can calculate the motion using Newton’s second law. The program that you wil ...
Energy - isd194 cms .demo. ties .k12. mn .us
... standing on a platform that is 10 m off the ground? 500 N x 10 m 5000 J ...
... standing on a platform that is 10 m off the ground? 500 N x 10 m 5000 J ...
S8P2 Students will be familiar with the forms and transformations of
... • Series and parallel circuits can be used to control the amount of electrical energy produced. • Every object exerts gravitational force on every other object. The force depends on the mass of the objects and the distance between them. ...
... • Series and parallel circuits can be used to control the amount of electrical energy produced. • Every object exerts gravitational force on every other object. The force depends on the mass of the objects and the distance between them. ...
The Law of Conservation of Energy
... energy when you are on a swing… At what point do you have the most potential energy? At what point do you have the most kinetic energy? What happens to the mechanical energy? ...
... energy when you are on a swing… At what point do you have the most potential energy? At what point do you have the most kinetic energy? What happens to the mechanical energy? ...
Types of Energy
... ●All matter is made up of atoms (particles) that move faster when they heat up. The faster the particles move, the higher the temperature. ●Heat energy is the transfer of thermal energy. ●Heat energy always moves from hotter objects to cooler objects. Radiant energy ●Energy which is transferred thro ...
... ●All matter is made up of atoms (particles) that move faster when they heat up. The faster the particles move, the higher the temperature. ●Heat energy is the transfer of thermal energy. ●Heat energy always moves from hotter objects to cooler objects. Radiant energy ●Energy which is transferred thro ...
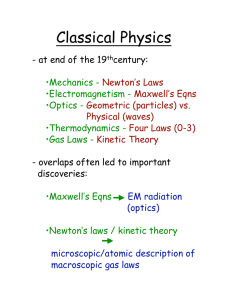
Classical Physics
... Heat and Thermodynamics - study of Thermal Energy of systems Temperature: a measure of thermal energy, units of Kelvins Room Temp ~ 290 K Temperature of an object is measured by the change in some physical property. Measuring device is called a thermometer. ...
... Heat and Thermodynamics - study of Thermal Energy of systems Temperature: a measure of thermal energy, units of Kelvins Room Temp ~ 290 K Temperature of an object is measured by the change in some physical property. Measuring device is called a thermometer. ...
File
... How high will the ball rise? (Use Conservation of Energy to solve!) (20.4 m) 2. A sled is at the top of a 40 meter high hill. The sled and its rider have a mass of 70 kg. a. What is the GPE of sled and rider? (27,440 J) b. How fast will the sled be moving at the bottom of the hill? (28 m/s) c. If th ...
... How high will the ball rise? (Use Conservation of Energy to solve!) (20.4 m) 2. A sled is at the top of a 40 meter high hill. The sled and its rider have a mass of 70 kg. a. What is the GPE of sled and rider? (27,440 J) b. How fast will the sled be moving at the bottom of the hill? (28 m/s) c. If th ...
The Origin of the Equation for Escape Speed To understand where
... in which G is the universal gravitational constant and the units of the potential energy are Joules if the other quantities are expressed in standard units. The minus sign in this equation reflects the fact that setting the zero point of potential energy is like picking the zero point of a coordinat ...
... in which G is the universal gravitational constant and the units of the potential energy are Joules if the other quantities are expressed in standard units. The minus sign in this equation reflects the fact that setting the zero point of potential energy is like picking the zero point of a coordinat ...
Energy 1
... • Temperature is related to the average kinetic energy of an object’s atoms or molecules. ...
... • Temperature is related to the average kinetic energy of an object’s atoms or molecules. ...
Golden Valley HS • AP Chemistry
... energy cannot be created or destroyed, it is always conserved. This is also referred to as the First Law of Thermodynamics. The system is the part of the universe that is under study. Everything not part of the system is considered the surroundings. An open system can transfer energy and matter to a ...
... energy cannot be created or destroyed, it is always conserved. This is also referred to as the First Law of Thermodynamics. The system is the part of the universe that is under study. Everything not part of the system is considered the surroundings. An open system can transfer energy and matter to a ...
PE and KE
... it can only be changed from one of form of energy to another form. • The amount of energy stays the same. ...
... it can only be changed from one of form of energy to another form. • The amount of energy stays the same. ...
Laws of Thermodynamics - Ohio Wesleyan University
... Temperatures must be in Kelvin All Carnot engines operating between the same two temperatures will have the same efficiency The efficiency increases as Tc is lowered and as Th is raised Efficiency is 0 if Th = Tc Efficiency is 100% only if Tc = 0 K (not possible from third law of thermodynamics) In ...
... Temperatures must be in Kelvin All Carnot engines operating between the same two temperatures will have the same efficiency The efficiency increases as Tc is lowered and as Th is raised Efficiency is 0 if Th = Tc Efficiency is 100% only if Tc = 0 K (not possible from third law of thermodynamics) In ...
Electrical Energy
... electrical energy is generated using renewable and nonrenewable resources. Students will identify several ways in which energy may be stored. Students will be able to compare how mechanical to electrical energy and electrical to thermal energy is transformed. Students will be able to explain how the ...
... electrical energy is generated using renewable and nonrenewable resources. Students will identify several ways in which energy may be stored. Students will be able to compare how mechanical to electrical energy and electrical to thermal energy is transformed. Students will be able to explain how the ...