* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Golden Valley HS • AP Chemistry
Dynamic insulation wikipedia , lookup
Temperature wikipedia , lookup
Thermal radiation wikipedia , lookup
Internal energy wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat capacity wikipedia , lookup
Conservation of energy wikipedia , lookup
Calorimetry wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
R-value (insulation) wikipedia , lookup
Countercurrent exchange wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Adiabatic process wikipedia , lookup
Thermodynamic system wikipedia , lookup
Heat equation wikipedia , lookup
Heat transfer physics wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
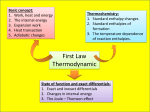
Golden Valley HS • AP Chemistry [Keep for Reference] 5 • Thermochemistry Basics A BLUFFER’S GUIDE The Law of Conservation of Energy says that energy cannot be created or destroyed, it is always conserved. This is also referred to as the First Law of Thermodynamics. The system is the part of the universe that is under study. Everything not part of the system is considered the surroundings. An open system can transfer energy and matter to and from the surroundings. A closed system is where energy can be transferred to the surroundings, but matter cannot. State functions depend only on the difference between the final and initial state of the system. The path to the state of the system does not matter. E = change in energy (state function) q = heat, enthalpy (not a state function) w = work (not a state function) H = change in heat, heat of rxn (state function) S = change in entropy (state function) G = change in Gibb’s free energy (state function) When H or q is positive (+), the reaction is endothermic, because it is absorbing heat from the surroundings therefore they feel cool or cold. When H or q is negative (–), the reaction is exothermic, releasing heat to the surroundings and feel warm or hot. Heat can be defined in calories, which is the amount of heat needed to raise 1 gram of H2O 1oC. The joule is the SI unit of energy. 1 calorie = 4.184 J This is also the specific heat of water (4.184 J/goC). Specific heat of other substances is defined as the energy needed to raise 1 gram of the substance 1oC. Substances with larger specific heat values take more energy to change the temperature. They also hold their heat longer. Water has a very high specific heat, whereas metals have low specific heats. q = mCT m is the mass, C is the specific heat (sometimes abbreviated as Cp) and T is the change in temperature (final temperature – initial temperature). The temperature can be measured in Kelvin or Celsius. Calorimetry is the measurement of heat flow, it is measured using a calorimeter. These can be fancy (bomb calorimeter – a sealed metal cup where a material can be combusted) or simple (coffee cup calorimeter). Enthalpies of Reaction or Heat of Reaction (Hrxn) measures the change in enthalpy (heat) of the given reaction. It is measured by: H = Hproducts – Hreactants This is also known as “final minus initial.” The heats involved needed to be provided using the heats of formation in Appendix C. Note: the Hfo for elements is 0. Hess’s Law says that if a reaction is carried out in a series of steps, H for the overall reaction will equal the sum of the enthalpy changes for the individual steps. What you do mathematically to the operation you must also do the Hrxn. 1. If the coefficients of a chemical reaction are all multiplied by a constant, the Hrxn is multiplied by that same constant. 2. If two or more reactions are added together to obtain an overall reaction, the heats of these reactions are also added to give the heat of the overall reaction. 3. If a reaction is reversed, then the Hrxn must also have its sign changed to its opposite.