What are magic numbers? - Justus-Liebig
... Explanation of the magic numbers • the use of the Wood-Saxon potential provides a shift and splitting of the terms of the oscillator potential • taking into account the spin-orbit coupling provides a further breakdown and creates gaps in the term scheme to match the magic numbers • the spins and or ...
... Explanation of the magic numbers • the use of the Wood-Saxon potential provides a shift and splitting of the terms of the oscillator potential • taking into account the spin-orbit coupling provides a further breakdown and creates gaps in the term scheme to match the magic numbers • the spins and or ...
AP Chemistry Test Review
... 46) know the signs for ∆S, ∆G, and ∆H and when each of the values are zero 47) spontaneous reactions have −∆G or + E°cell 48) ∆G° = zero for pure elements in their standard state 49) LEO- ANO; CPR-GER…how to balance redox reactions and find ox. agents or red. agents 50) calculate E°cell and be able ...
... 46) know the signs for ∆S, ∆G, and ∆H and when each of the values are zero 47) spontaneous reactions have −∆G or + E°cell 48) ∆G° = zero for pure elements in their standard state 49) LEO- ANO; CPR-GER…how to balance redox reactions and find ox. agents or red. agents 50) calculate E°cell and be able ...
chapter 7 quiz
... 10._T__The number of protons and neutrons in the nucleus L) cathode of an atom. M) electron cloud 11._Y__Discovered radioactivity. N) Darth Vader 12._C__Discovered three types of radiation. O) chemical symbol 13._J__The charge on an “beta” particle. P) 0 14._A__The charge on an “alpha” particle. Q) ...
... 10._T__The number of protons and neutrons in the nucleus L) cathode of an atom. M) electron cloud 11._Y__Discovered radioactivity. N) Darth Vader 12._C__Discovered three types of radiation. O) chemical symbol 13._J__The charge on an “beta” particle. P) 0 14._A__The charge on an “alpha” particle. Q) ...
Lecture 12 Atomic structure
... Since single-particle Hamiltonian Ĥ0 continues to commute with the angular momentum operator, [Ĥ0 , L̂] = 0, its eigenfunctions remain indexed by quantum numbers (n, #, m! , ms ). However, since effective potential, V (r ) + Ui (r ), is no longer Coulomb-like, # values for a given n need not be de ...
... Since single-particle Hamiltonian Ĥ0 continues to commute with the angular momentum operator, [Ĥ0 , L̂] = 0, its eigenfunctions remain indexed by quantum numbers (n, #, m! , ms ). However, since effective potential, V (r ) + Ui (r ), is no longer Coulomb-like, # values for a given n need not be de ...
January 2005
... Consider wave propagation in a one-dimensional medium which consists of a large number of pendula of mass m and length l coupled by springs of spring constant K. The distance between adjacent masses is a0 , which is also the natural length of the springs. ...
... Consider wave propagation in a one-dimensional medium which consists of a large number of pendula of mass m and length l coupled by springs of spring constant K. The distance between adjacent masses is a0 , which is also the natural length of the springs. ...
Chemistry Review - pams-hoey
... nucleus 2. Radioactive decay: The spontaneous breakdown of an unstable atomic nucleus 3. Decay Series: The series of steps by which a radioactive nucleus decays into a non-radioactive nucleus. 4. Alpha Decay: Occurs when a nucleus releases an alpha particle 5. Beta Decay: Loses a beta particle causi ...
... nucleus 2. Radioactive decay: The spontaneous breakdown of an unstable atomic nucleus 3. Decay Series: The series of steps by which a radioactive nucleus decays into a non-radioactive nucleus. 4. Alpha Decay: Occurs when a nucleus releases an alpha particle 5. Beta Decay: Loses a beta particle causi ...
The Quantum Model of the Atom
... Heisenberg Uncertainty Principle: it is impossible to determine simultaneously both the position and velocity of an electron or any other particle ...
... Heisenberg Uncertainty Principle: it is impossible to determine simultaneously both the position and velocity of an electron or any other particle ...
A spectral theoretic approach to quantum
... • In fact, we prove that H is integrable in a stronger sense: it is equivalent (via change of orthonormal basis) to an integrable, canonically quantized, smooth classical ndimensional Hamiltonian over , set into Birkhoff’s normal form. Thus, in this basis we have separation of variables in the sense ...
... • In fact, we prove that H is integrable in a stronger sense: it is equivalent (via change of orthonormal basis) to an integrable, canonically quantized, smooth classical ndimensional Hamiltonian over , set into Birkhoff’s normal form. Thus, in this basis we have separation of variables in the sense ...
1) - Kurt Niedenzu
... 32) The increase in atomic radius of each successive element within a group is primarily due to an increase in the number of a) neutrons in the nucleus b) electrons in the outermost shell c) unpaired electrons d) occupied principal energy levels 33) Elements that have properties of both metals and n ...
... 32) The increase in atomic radius of each successive element within a group is primarily due to an increase in the number of a) neutrons in the nucleus b) electrons in the outermost shell c) unpaired electrons d) occupied principal energy levels 33) Elements that have properties of both metals and n ...
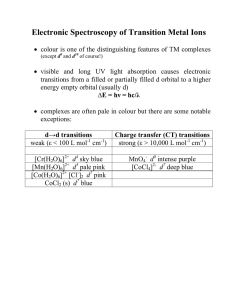
Electronic Spectroscopy of Transition Metal Ions
... • combine these into S and L which represent the TOTAL spin and orbital angular momentum • there are many different arrangements of electrons in d orbitals so this gives rise to many possible states (RussellSaunders ‘terms’) that represent different energies for the system as a whole eg. d2 ion 1st ...
... • combine these into S and L which represent the TOTAL spin and orbital angular momentum • there are many different arrangements of electrons in d orbitals so this gives rise to many possible states (RussellSaunders ‘terms’) that represent different energies for the system as a whole eg. d2 ion 1st ...
PPT - Henry Haselgrove`s Homepage
... Entanglement, correlation, and errorcorrection in the ground states of manybody systems ...
... Entanglement, correlation, and errorcorrection in the ground states of manybody systems ...