Particles and interactions
... •classify particles according to their spin; •understand the Pauli exclusion principle and how it is applied; •understand and apply the Heisenberg uncertainty principle for energy and time; •appreciate the meaning of the term virtual particle, •describe the fundamental interactions; •state the meani ...
... •classify particles according to their spin; •understand the Pauli exclusion principle and how it is applied; •understand and apply the Heisenberg uncertainty principle for energy and time; •appreciate the meaning of the term virtual particle, •describe the fundamental interactions; •state the meani ...
Control of size of nano / micro particles synthesized by atmospheric
... M.V. Mishin, K.Y. Zamotin, A.S. Kondrateva, V.S. Protopopova. Investigation of various nano-objects formation is important basement of development of micro and nano-electronics and other important technologies. Nanoparticles formed from different materials are already used in modern techniques to ta ...
... M.V. Mishin, K.Y. Zamotin, A.S. Kondrateva, V.S. Protopopova. Investigation of various nano-objects formation is important basement of development of micro and nano-electronics and other important technologies. Nanoparticles formed from different materials are already used in modern techniques to ta ...
Quantum Mechanics
... Thus, Y2 is the probability of finding a certain electron at the given position and time The Y2 function gives us the shapes of the orbitals ...
... Thus, Y2 is the probability of finding a certain electron at the given position and time The Y2 function gives us the shapes of the orbitals ...
Artificial Transmutation
... Since Rutherford, Scientists have made hundreds of new isotopes AND new elements - All elements above atomic no. 92 are called ...
... Since Rutherford, Scientists have made hundreds of new isotopes AND new elements - All elements above atomic no. 92 are called ...
PROBLEM 1 [25 PTS] A system consists of N distinquishable
... a) In terms of the velocity components vx and vy , how many particles are there with vx in the infinitessimal range between vx and vx + dvx , and in the infinitessimal range between vy and vy + dvy ? Make sure that you use the formulas for gaussian integrals from the equation sheet to normalize the ...
... a) In terms of the velocity components vx and vy , how many particles are there with vx in the infinitessimal range between vx and vx + dvx , and in the infinitessimal range between vy and vy + dvy ? Make sure that you use the formulas for gaussian integrals from the equation sheet to normalize the ...
ELEMENTS Using Textbk (pg 172-173) or Planner make flashcards
... ALLOTROPE and EFFUSION and DIFFUSION: (1) ALLOTROPE is a different form of the an element in the same physical state of matter (a) it is “1” type of atom, but the atoms are arranged differently, which give the allotropes different properties (b) each allotrope is a pure form of that element 1. (e.g ...
... ALLOTROPE and EFFUSION and DIFFUSION: (1) ALLOTROPE is a different form of the an element in the same physical state of matter (a) it is “1” type of atom, but the atoms are arranged differently, which give the allotropes different properties (b) each allotrope is a pure form of that element 1. (e.g ...
\chapter{Introduction}
... particles whatsoever or, more rigorously, a subspace $V$ of the $\mathbb{R}^3$ such that $N(V)=0$, where $N$ denotes the number of particles detected by an observer in the exterior of $V$. Intuitively this function $N:\mathbb{R}^3\rightarrow\mathbb{N}$ is an invariant under coordinate transformation ...
... particles whatsoever or, more rigorously, a subspace $V$ of the $\mathbb{R}^3$ such that $N(V)=0$, where $N$ denotes the number of particles detected by an observer in the exterior of $V$. Intuitively this function $N:\mathbb{R}^3\rightarrow\mathbb{N}$ is an invariant under coordinate transformation ...
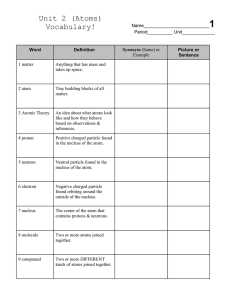
Unit 6 Science Vocabulary Emily 6th
... 5. an arrangement of the elements in order of their atomic numbers such that elements with similar properties fall in the same group 6. the smallest particle into which an element can be divided. 7. uncharged particles that are found within the nucleus of an atom. 8. the center of an atom made up of ...
... 5. an arrangement of the elements in order of their atomic numbers such that elements with similar properties fall in the same group 6. the smallest particle into which an element can be divided. 7. uncharged particles that are found within the nucleus of an atom. 8. the center of an atom made up of ...
Chapter 2 Study Guide
... 1. The first subatomic particle discovered was ___________________________. 2. The only subatomic particle that does not carry an electric charge is the __________. 3. The atomic number of an element whose atoms have 12 protons and 11 neutrons is _____. 4. The mass number of an element whose atoms h ...
... 1. The first subatomic particle discovered was ___________________________. 2. The only subatomic particle that does not carry an electric charge is the __________. 3. The atomic number of an element whose atoms have 12 protons and 11 neutrons is _____. 4. The mass number of an element whose atoms h ...
lect22
... variables” which we do not know but which gives the system an underlying deterministic structure. We hide our ignorance by describing the “most probable” outcomes of measurement ...
... variables” which we do not know but which gives the system an underlying deterministic structure. We hide our ignorance by describing the “most probable” outcomes of measurement ...










![PROBLEM 1 [25 PTS] A system consists of N distinquishable](http://s1.studyres.com/store/data/006063913_1-e1778e5c6114fd66466f556bb5f30c03-300x300.png)