Problem Set 1 - MIT OpenCourseWare
... 2. (25 points) Dimensional Analysis: Two Kinds of Quantum Gravity (a) Gravitational bound states Consider a particle sitting on a table which is kept from floating away only by the force of gravity. This system is characterized by just three physical parameters, the mass of the particle, m, the acce ...
... 2. (25 points) Dimensional Analysis: Two Kinds of Quantum Gravity (a) Gravitational bound states Consider a particle sitting on a table which is kept from floating away only by the force of gravity. This system is characterized by just three physical parameters, the mass of the particle, m, the acce ...
PowerPoint Presentation - Particle Physics Group
... Most Particles are unstable – I.e they decay. Important property is the lifetime of the particle Quantum Mechanical Effect – Decay is random We have to measure many decays and take the average to determine a real lifetime (in fact we need to fit the data) ...
... Most Particles are unstable – I.e they decay. Important property is the lifetime of the particle Quantum Mechanical Effect – Decay is random We have to measure many decays and take the average to determine a real lifetime (in fact we need to fit the data) ...
Many-body Quantum Mechanics
... the method of using annihilation and creation operators acting on a Fock space as ”second quantization”. As should be clear from the above, this terminology is misleading in the sense that ψ̂ is not a once more quantized version of the wave function, but an object which is directly (or via a Fourier ...
... the method of using annihilation and creation operators acting on a Fock space as ”second quantization”. As should be clear from the above, this terminology is misleading in the sense that ψ̂ is not a once more quantized version of the wave function, but an object which is directly (or via a Fourier ...
AP Physics Daily Problem #107
... located 0.3m apart as shown here. Note that the lower charge is negative. Draw your estimate of the net force vector on each particle. ...
... located 0.3m apart as shown here. Note that the lower charge is negative. Draw your estimate of the net force vector on each particle. ...
6.1.1
... • They conceptualized the building blocks of matter – which they called the ‘atom’ -- literally means ‘cannot be cut’. ...
... • They conceptualized the building blocks of matter – which they called the ‘atom’ -- literally means ‘cannot be cut’. ...
BWilliamsLtalk - FSU High Energy Physics
... Quantum mechanics can only tell you the statistical probabilities of certain outcomes or positions, not because of any flaw in the theory, but because this is the way ...
... Quantum mechanics can only tell you the statistical probabilities of certain outcomes or positions, not because of any flaw in the theory, but because this is the way ...
Notes. - Net Start Class
... charged mixture of ions and electrons. Particles that have been ionized (charged) There exist at extremely high temperatures. Exist at naturally (stars) or man-made (neon signs). While plasma is the most abundant phase of matter in the universe, on earth it only occurs in a few limited places. ...
... charged mixture of ions and electrons. Particles that have been ionized (charged) There exist at extremely high temperatures. Exist at naturally (stars) or man-made (neon signs). While plasma is the most abundant phase of matter in the universe, on earth it only occurs in a few limited places. ...
Electrical Force - Scarsdale Schools
... mass 9.1 x 10^-31 kg and charge q = 1.6 x 10^-19 C. Suppose a positron moves in the vicinity of an (alpha) particle, which has charge 3.2 x 10^-19 C. The alpha particle’s mass is more than 7000 times that of the positron, so we assume that the particle remains at rest. ...
... mass 9.1 x 10^-31 kg and charge q = 1.6 x 10^-19 C. Suppose a positron moves in the vicinity of an (alpha) particle, which has charge 3.2 x 10^-19 C. The alpha particle’s mass is more than 7000 times that of the positron, so we assume that the particle remains at rest. ...
Problem Set 10
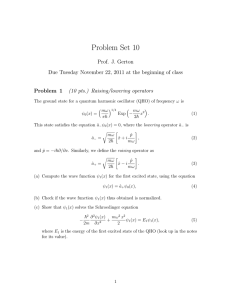
... coming from x = −∞. (a) Write down the wave function for x < 0. Here, are there left- and right-moving components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and right-moving components of the wavefunction? Why? (c) Write down the boundary conditions a ...
... coming from x = −∞. (a) Write down the wave function for x < 0. Here, are there left- and right-moving components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and right-moving components of the wavefunction? Why? (c) Write down the boundary conditions a ...
$doc.title
... c. What is the radiation force on each free electron, in terms of the quasar luminosity L ? The radiation force is exerted via Thomson scattering of photons on free electrons (cross section σ Τ ). ...
... c. What is the radiation force on each free electron, in terms of the quasar luminosity L ? The radiation force is exerted via Thomson scattering of photons on free electrons (cross section σ Τ ). ...
AP Physics Daily Problem #120
... A capacitor consists of two metal plates of diameter 50cm separated by a gap of 0.5mm. It is placed in a vacuum. 0.5mm ...
... A capacitor consists of two metal plates of diameter 50cm separated by a gap of 0.5mm. It is placed in a vacuum. 0.5mm ...
notes - UBC Physics
... of interest and, at least in the case of non-interacting field theories, to analyze the physical properties of the particle states that arise quantum mechanically. In general, the procedure goes like this: • Identify the degrees of freedom and symmetries (or conserved quantities) of the physical sys ...
... of interest and, at least in the case of non-interacting field theories, to analyze the physical properties of the particle states that arise quantum mechanically. In general, the procedure goes like this: • Identify the degrees of freedom and symmetries (or conserved quantities) of the physical sys ...
Particles and Waves
... Again, when measured accurately, the sum of the masses of the particles produced by the reaction is actually slightly less than the sum of masses of the particles before the reaction and this ‘lost mass’ is turned into energy according to Einstein’s mass-energy equivalence principle (E=mc2). Nuclear ...
... Again, when measured accurately, the sum of the masses of the particles produced by the reaction is actually slightly less than the sum of masses of the particles before the reaction and this ‘lost mass’ is turned into energy according to Einstein’s mass-energy equivalence principle (E=mc2). Nuclear ...
Chemistry
... All atoms consist of even smaller particles—protons, neutrons, and electrons. The center of an atom is called the nucleus, which is made up of protons and neutrons. A proton is a tiny particle that has mass and a positive electric charge. A neutron is a tiny particle with approximately the same mas ...
... All atoms consist of even smaller particles—protons, neutrons, and electrons. The center of an atom is called the nucleus, which is made up of protons and neutrons. A proton is a tiny particle that has mass and a positive electric charge. A neutron is a tiny particle with approximately the same mas ...
Entanglement of Identical Particles
... Entanglement of Identical Particles In quantum entanglement, two particles are correlated in such a way that any action on one of them affects the other even when they are far apart. The traditional methods of measuring the degree of quantum entanglement were originally developed for nonidentical pa ...
... Entanglement of Identical Particles In quantum entanglement, two particles are correlated in such a way that any action on one of them affects the other even when they are far apart. The traditional methods of measuring the degree of quantum entanglement were originally developed for nonidentical pa ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.