February 4
... concentric shells around the nucleus • Innermost shell can contain 2 electrons • The second and third shells can contain 8 electrons each • The fourth and fifth shells can contain 18 electrons each ...
... concentric shells around the nucleus • Innermost shell can contain 2 electrons • The second and third shells can contain 8 electrons each • The fourth and fifth shells can contain 18 electrons each ...
Mysteries of Mass Article in Scientific American
... people think they know what mass is, but they understand only part of the story. For instance, an elephant is clearly bulkier and weighs more than an ant. Even in the absence of gravity, the elephant would have greater mass— it would be harder to push and set in motion. Obviously the elephant is mo ...
... people think they know what mass is, but they understand only part of the story. For instance, an elephant is clearly bulkier and weighs more than an ant. Even in the absence of gravity, the elephant would have greater mass— it would be harder to push and set in motion. Obviously the elephant is mo ...
the view from noninertial frames
... A free particle is one that has no real forces acting on it; Newton's Law of Inertia says that a free particle will have constant velocity and hence zero acceleration. With respect to a non-inertial frame, however, a free particle does not move with constant velocity. Therefore, to use a noninertial ...
... A free particle is one that has no real forces acting on it; Newton's Law of Inertia says that a free particle will have constant velocity and hence zero acceleration. With respect to a non-inertial frame, however, a free particle does not move with constant velocity. Therefore, to use a noninertial ...
Words for Matter Chapter 2
... describes how matter changes when it reacts with other matter – For example, does it burn? Will it rust? Will it react with acid? Will it rot? is a material that always has the same makeup and properties : smallest particle of an element that has the same properties of that element : center of the a ...
... describes how matter changes when it reacts with other matter – For example, does it burn? Will it rust? Will it react with acid? Will it rot? is a material that always has the same makeup and properties : smallest particle of an element that has the same properties of that element : center of the a ...
Quantum Theory
... Events can happen without a force or signal to cause it to happen the fabric of space allows, or even causes it to happen. Objects do not always have specific properties until they are interacted with; the properties hang in some sort of limbo. ...
... Events can happen without a force or signal to cause it to happen the fabric of space allows, or even causes it to happen. Objects do not always have specific properties until they are interacted with; the properties hang in some sort of limbo. ...
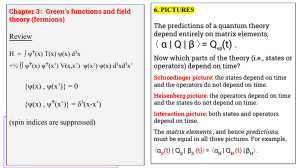
α | Q | β 〉= Q (t) . 〈 Review
... Perturbation theory and the interaction picture ... Assume H = H0 + H1 , where H0 is solvable and H1 is a set of interactions, possibly having small effects. {Usually H0 is a single particle operator; and H1 is a two-particle operator describing the interactions between particles.} ...
... Perturbation theory and the interaction picture ... Assume H = H0 + H1 , where H0 is solvable and H1 is a set of interactions, possibly having small effects. {Usually H0 is a single particle operator; and H1 is a two-particle operator describing the interactions between particles.} ...
here - University of Kent
... We calculate Skyrmions by solving complicated equations on the COSMOS supercomputer at DAMTP, University of Cambridge. ...
... We calculate Skyrmions by solving complicated equations on the COSMOS supercomputer at DAMTP, University of Cambridge. ...
The relation of colour charge to electric charge (E/c) −P2 −Q2 −(mc
... be factored into two linear parts using 4x4 Dirac matrices. [Dirac, P.A.M., The Principles of Quantum Mechanics, 4th edition (Oxford University Press) ISBN 0-19-852011-5] This can also be done using 2x2 Pauli matrices (labelled K,L,M) because two inertial observers agree on the component of momentum ...
... be factored into two linear parts using 4x4 Dirac matrices. [Dirac, P.A.M., The Principles of Quantum Mechanics, 4th edition (Oxford University Press) ISBN 0-19-852011-5] This can also be done using 2x2 Pauli matrices (labelled K,L,M) because two inertial observers agree on the component of momentum ...
PPT - Lawless Teaching : Home
... This is also the smallest amount of charge in nature and all other charges are integer multiples of this (we will later see quarks as the exception to this). Named by an Irish guy, George J. Stoney (cause that’s important). ...
... This is also the smallest amount of charge in nature and all other charges are integer multiples of this (we will later see quarks as the exception to this). Named by an Irish guy, George J. Stoney (cause that’s important). ...
Introduction to Electromagnetism
... Vectors, derivatives Coordinate systems Force and momentum Energies ...
... Vectors, derivatives Coordinate systems Force and momentum Energies ...
CHAPTER 3 Atoms: The Building Blocks of Matter
... 5 Points in his theory – All matter is composed of extremely small particles called atoms – Atoms of a given element are identical in size, mass, and other properties – Atoms cannot be subdivided, created, or destroyed – Atoms of different elements combine in simple whole number ratios to form compo ...
... 5 Points in his theory – All matter is composed of extremely small particles called atoms – Atoms of a given element are identical in size, mass, and other properties – Atoms cannot be subdivided, created, or destroyed – Atoms of different elements combine in simple whole number ratios to form compo ...
2.1 Historical Development
... So Rutherford proposed that the electrons are revolving round the nucleus at extremely high speeds at great distances from the nucleus. The centrifugal force arising from this motion balances the force of electrostatic attraction. The electrons, therefore, do not fall into the nucleus. 2.4 Objectio ...
... So Rutherford proposed that the electrons are revolving round the nucleus at extremely high speeds at great distances from the nucleus. The centrifugal force arising from this motion balances the force of electrostatic attraction. The electrons, therefore, do not fall into the nucleus. 2.4 Objectio ...
No Slide Title - FSU High Energy Physics
... is looking for the smallest constituents of matter (the “ultimate building blocks”) and for the fundamental forces between them; aim is to find description in terms of the smallest number of particles and forces (“interactions”) at given length scale, it is useful to describe matter in terms of spec ...
... is looking for the smallest constituents of matter (the “ultimate building blocks”) and for the fundamental forces between them; aim is to find description in terms of the smallest number of particles and forces (“interactions”) at given length scale, it is useful to describe matter in terms of spec ...
$doc.title
... F(q ) = ∫ ρ (r)e d r with ∫ ρ (r)d 3r = 1 ◆ In this simple model we could learn about an unknown charge distribution (structure) by measuring how many scatters occur in an angular region and comparing this measurement with what is expected for a “point charge”, |F(q2)| =1 and our favorite theoretic ...
... F(q ) = ∫ ρ (r)e d r with ∫ ρ (r)d 3r = 1 ◆ In this simple model we could learn about an unknown charge distribution (structure) by measuring how many scatters occur in an angular region and comparing this measurement with what is expected for a “point charge”, |F(q2)| =1 and our favorite theoretic ...
Lecture 1 - UW Canvas
... particle of smoke or soot. These are about 1 m in diameter, barely at the resolution limit of most microscopes. A particle of this size with the density of carbon has a mass of about 10-18 kg. What is the de Broglie wavelength for such a particle, if it is moving slowly at 1 mm/s? ...
... particle of smoke or soot. These are about 1 m in diameter, barely at the resolution limit of most microscopes. A particle of this size with the density of carbon has a mass of about 10-18 kg. What is the de Broglie wavelength for such a particle, if it is moving slowly at 1 mm/s? ...
Rutherford`s Gold Foil Experiment
... Rutherford is typically credited with the discovery of the proton in 1919. James Chadwick is credited with the discovery of the neutron in 1932 Proton has a positive charge equal to the magnitude of the negative charge of the electron. ...
... Rutherford is typically credited with the discovery of the proton in 1919. James Chadwick is credited with the discovery of the neutron in 1932 Proton has a positive charge equal to the magnitude of the negative charge of the electron. ...
Helical Particle Waves
... Gravitons and Inertial Mass The more energy a relativistic particle absorbs, the higher its spin moment becomes and thus gains higher resistance to directional change. That is, a stream of relativistic particles (a helical particle wave) starts to lose amplitude as the velocity at which it propagate ...
... Gravitons and Inertial Mass The more energy a relativistic particle absorbs, the higher its spin moment becomes and thus gains higher resistance to directional change. That is, a stream of relativistic particles (a helical particle wave) starts to lose amplitude as the velocity at which it propagate ...
PowerPoint ******
... discovery of a new particle with a mass between 125 and 127 GeV/c2 was announced; physicists suspected that it was the Higgs boson. By March 2013, the particle had been proven to behave, interact and decay in many of the ways predicted by the Standard Model, and was also tentatively confirmed to hav ...
... discovery of a new particle with a mass between 125 and 127 GeV/c2 was announced; physicists suspected that it was the Higgs boson. By March 2013, the particle had been proven to behave, interact and decay in many of the ways predicted by the Standard Model, and was also tentatively confirmed to hav ...
power point notes
... Rutherford proposed that the atom consists of a tiny positively charged nucleus surrounded by a cloud of negatively charged electrons. The nucleus contains almost all of the mass of the atom and consists of protons and neutrons. The number of electrons surrounding the nucleus, equals the number of p ...
... Rutherford proposed that the atom consists of a tiny positively charged nucleus surrounded by a cloud of negatively charged electrons. The nucleus contains almost all of the mass of the atom and consists of protons and neutrons. The number of electrons surrounding the nucleus, equals the number of p ...
Section 13.2 - CPO Science
... 13.2 Bohr model of the atom • Danish physicist Neils Bohr proposed the concept of energy levels to explain the spectrum of hydrogen. • When an electron moves from a higher energy level to a lower one, the atom gives up the energy difference between the two levels. • The energy comes out as differen ...
... 13.2 Bohr model of the atom • Danish physicist Neils Bohr proposed the concept of energy levels to explain the spectrum of hydrogen. • When an electron moves from a higher energy level to a lower one, the atom gives up the energy difference between the two levels. • The energy comes out as differen ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.