Astronomy Preview Name: Worksheet 1 Block: Match the definitio
... A. The force generated by electric charges B. Matter that emits no detectable radiation but whose presence can be deduced by its gravitational attraction on other bodies C. A subatomic particle of nearly the same mass as the proton but with no electric charge D. A body in orbit around a star that is ...
... A. The force generated by electric charges B. Matter that emits no detectable radiation but whose presence can be deduced by its gravitational attraction on other bodies C. A subatomic particle of nearly the same mass as the proton but with no electric charge D. A body in orbit around a star that is ...
Quantum Notes
... • The wave model of light cannot explain all of light’s characteristics; Albert Einstein proposed in 1905 that light has a dual nature (wave-like and particle-like) • Matter can gain or lose energy only in small, specific amounts called quanta •A quantum is the minimum amount of energy that can be g ...
... • The wave model of light cannot explain all of light’s characteristics; Albert Einstein proposed in 1905 that light has a dual nature (wave-like and particle-like) • Matter can gain or lose energy only in small, specific amounts called quanta •A quantum is the minimum amount of energy that can be g ...
THE HISTORY OF THE ATOM Table of Contents Black Boxes
... But, in addition to the negatively charged electrons, there must be something giving it a positive charge because the overall charge of the atom is neutral (not negative) Let’s look at Thomson’s model of the atom… Thomson’s Plum Pudding Model of the Atom He believed the atom was made of positively c ...
... But, in addition to the negatively charged electrons, there must be something giving it a positive charge because the overall charge of the atom is neutral (not negative) Let’s look at Thomson’s model of the atom… Thomson’s Plum Pudding Model of the Atom He believed the atom was made of positively c ...
Chapter 24: Electric Potential
... The electric potential at points in an xy plane is given by V = (2.0x2 – 3.0y2) V/m2. In unit-vector notation, what is the electric field at the point (3.0 m, 2.0 m)? ...
... The electric potential at points in an xy plane is given by V = (2.0x2 – 3.0y2) V/m2. In unit-vector notation, what is the electric field at the point (3.0 m, 2.0 m)? ...
Statistical laws
... etc.) are the average of motions of many molecules. Within the framework of classic mechanics, one needs to solve Newton’s equations of 6×1023 interacting molecules. This is obviously not possible and also unnecessary. The reason is that the presence of a large number of particles causes new types ...
... etc.) are the average of motions of many molecules. Within the framework of classic mechanics, one needs to solve Newton’s equations of 6×1023 interacting molecules. This is obviously not possible and also unnecessary. The reason is that the presence of a large number of particles causes new types ...
Wave Particle Duality File
... • Planck had found that light under certain circumstances behaved as a stream of particles called photons. • These particles are as real as electrons. • The wave and particle nature of light are ...
... • Planck had found that light under certain circumstances behaved as a stream of particles called photons. • These particles are as real as electrons. • The wave and particle nature of light are ...
Beryllium isotopes in geochronology Cosmogenic Be and Be
... fission – the spontaneous (or induced by particle collision) splitting of a heavy nucleus into a pair (only rarely more) of nearly equal fission fragments (fission products) generally with some neutrons. Fission is accompanied by the release of a large quantity of energy. [return] gamma rays (gamma ...
... fission – the spontaneous (or induced by particle collision) splitting of a heavy nucleus into a pair (only rarely more) of nearly equal fission fragments (fission products) generally with some neutrons. Fission is accompanied by the release of a large quantity of energy. [return] gamma rays (gamma ...
bring the rain - Black Dog Music Studio
... Accelerator Laboratory (Fermilab). This is the home to an atomic particle accelerator where atoms are violently slammed into each other at indescribable speeds and a collision detection center takes “pictures” of the results. Among the many results recorded at Fermilab are a type of subatomic partic ...
... Accelerator Laboratory (Fermilab). This is the home to an atomic particle accelerator where atoms are violently slammed into each other at indescribable speeds and a collision detection center takes “pictures” of the results. Among the many results recorded at Fermilab are a type of subatomic partic ...
Handout Topic 5 and 10 -11 NEW Selected Problems 3
... 8. The electromotive force (emf) of a cell is defined as A. the power supplied by the cell per unit current from the cell. B. the force that the cell provides to drive electrons round a circuit. C. the energy supplied by the cell per unit current from the cell. D. the potential difference across the ...
... 8. The electromotive force (emf) of a cell is defined as A. the power supplied by the cell per unit current from the cell. B. the force that the cell provides to drive electrons round a circuit. C. the energy supplied by the cell per unit current from the cell. D. the potential difference across the ...
Variance reduction in computations of neoclassical transport in
... is fixed and then iterations are repeated formally putting F = FM and Q = QM and applying the same procedure to the resulting integral equation. Due to annihilation and fixed module of the weight, the number of test particles needed for sampling the source term from the grid is decreasing and iterat ...
... is fixed and then iterations are repeated formally putting F = FM and Q = QM and applying the same procedure to the resulting integral equation. Due to annihilation and fixed module of the weight, the number of test particles needed for sampling the source term from the grid is decreasing and iterat ...
CHAPTER 3: The Experimental Basis of Quantum
... A positron passing through matter will likely annihilate with an electron. The electron and positron can form an atom-like configuration first, called positronium. Pair annihilation in empty space produces two photons to conserve momentum. Annihilation near a nucleus can result in a single photon. ...
... A positron passing through matter will likely annihilate with an electron. The electron and positron can form an atom-like configuration first, called positronium. Pair annihilation in empty space produces two photons to conserve momentum. Annihilation near a nucleus can result in a single photon. ...
Principles of Scientific Simulation
... – These give rise to different algorithms and in some cases, one will mix these different descriptions. We will briefly describe these with a pointer to types of algorithms used. – These descriptions underlie several different fields such as physics, chemistry, biology, environmental modeling, clima ...
... – These give rise to different algorithms and in some cases, one will mix these different descriptions. We will briefly describe these with a pointer to types of algorithms used. – These descriptions underlie several different fields such as physics, chemistry, biology, environmental modeling, clima ...
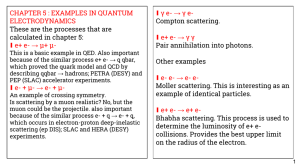
CHAPTER 5 : EXAMPLES IN QUANTUM γ e- → γ e- ∎ ELECTRODYNAMICS
... These were important experiments in the history of high-energy physics. From the particle data group, the figure shows the ratio R = σ ( e e → hadrons ) / σ ( e ebar → μ μbar) . The underlying process in hadron production is e- + e+ → q + qbar. Neglecting QCD interactions we would just have R = cons ...
... These were important experiments in the history of high-energy physics. From the particle data group, the figure shows the ratio R = σ ( e e → hadrons ) / σ ( e ebar → μ μbar) . The underlying process in hadron production is e- + e+ → q + qbar. Neglecting QCD interactions we would just have R = cons ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.