What lies beyond? - University of Toronto Physics
... strong coupling limits of gauge field theories; perhaps they are the same and in fact gravity = gauge theory! These dualities give us a new handle on (supersymmetric) strong-coupling gauge theory dynamics. ...
... strong coupling limits of gauge field theories; perhaps they are the same and in fact gravity = gauge theory! These dualities give us a new handle on (supersymmetric) strong-coupling gauge theory dynamics. ...
Lecture 13 : Diffusion equation / Transport (powerpoint)
... particles. No description is possible that allows for the determination of position and velocity of all these particles Only averaged quantities can be described. The evolution of the averaged velocity is however influenced by a microscopic process : the ...
... particles. No description is possible that allows for the determination of position and velocity of all these particles Only averaged quantities can be described. The evolution of the averaged velocity is however influenced by a microscopic process : the ...
Sep. 28 - Bryn Mawr College
... • Things have charge. This causes things to be attracted to and repelled by other things. This is called the electric force. It is one of four fundamental forces in nature (along with gravity, the weak force, and the strong force). Charge is labeled by q and has units of Coulombs. • Charge flowing t ...
... • Things have charge. This causes things to be attracted to and repelled by other things. This is called the electric force. It is one of four fundamental forces in nature (along with gravity, the weak force, and the strong force). Charge is labeled by q and has units of Coulombs. • Charge flowing t ...
The birth of quantum mechanics
... This energy spectrum was derived by Rayleigh and Jeans by assuming that blackbody radiation is emitted from atomic oscillators as a wave process, and that there must be detailed balance between the standing waves that can be supported inside the solid and the emitted radiation that comes out. ...
... This energy spectrum was derived by Rayleigh and Jeans by assuming that blackbody radiation is emitted from atomic oscillators as a wave process, and that there must be detailed balance between the standing waves that can be supported inside the solid and the emitted radiation that comes out. ...
Dark Matter and Energy: An Overview and Possible Solution
... are not baryons, and dark energy, which is the result of the cosmological term in Einstein’s field equations. Finding out what these materials are had been troublesome since the standard model of quantum mechanics doesn’t supply the non-relativistic particles needed for dark matter and since quantum ...
... are not baryons, and dark energy, which is the result of the cosmological term in Einstein’s field equations. Finding out what these materials are had been troublesome since the standard model of quantum mechanics doesn’t supply the non-relativistic particles needed for dark matter and since quantum ...
Physics - ideas about mythology and Greek Gods, and brain functions
... The measure of how much energy has dispersed is called a measure of “entropy.” Entropy is always increasing. This means that some energy is always lost as heat in any machine. When you convert the energy in a fuel to the energy in a machine, some joules of energy are always lost. The efficiency of a ...
... The measure of how much energy has dispersed is called a measure of “entropy.” Entropy is always increasing. This means that some energy is always lost as heat in any machine. When you convert the energy in a fuel to the energy in a machine, some joules of energy are always lost. The efficiency of a ...
Lecture 15 Summary
... the Grand Canonical Ensemble of statistical mechanics. We saw that temperature can be used to decide how energy will flow between two systems when they are brought into thermal contact. Simply put, the system with the higher temperature will spontaneously deliver energy to the system with the lower ...
... the Grand Canonical Ensemble of statistical mechanics. We saw that temperature can be used to decide how energy will flow between two systems when they are brought into thermal contact. Simply put, the system with the higher temperature will spontaneously deliver energy to the system with the lower ...
Basic Chemistry of Atoms
... The mass number (I do not like this term but I have to live with it) of an element is the sum of the protons and neutrons in a nucleus. The total number of nucleons of an atom is the mass number. IT IS NOT THE MASS OF AN ATOM. The atomic mass unit, abbreviated amu, is used for the mass of atoms sinc ...
... The mass number (I do not like this term but I have to live with it) of an element is the sum of the protons and neutrons in a nucleus. The total number of nucleons of an atom is the mass number. IT IS NOT THE MASS OF AN ATOM. The atomic mass unit, abbreviated amu, is used for the mass of atoms sinc ...
Jan. 26: Symmetries - Michigan State University
... state the transformation law of the quantized field. One will say, for instance, that a certain kind of spinless particles are the quanta of a "pseudoscalar" field, i.e., a. field y such that the transformation law for an inversion at the origin is ...
... state the transformation law of the quantized field. One will say, for instance, that a certain kind of spinless particles are the quanta of a "pseudoscalar" field, i.e., a. field y such that the transformation law for an inversion at the origin is ...
Answers
... In the early 60’s physicists detected hundreds of different ‘elementary’ particles formed by high energy collisions in particle accelerators using bubble chambers. These are large vessels of liquid hydrogen in a uniform magnetic field. Charged particles passing through the hydrogen leave a visible t ...
... In the early 60’s physicists detected hundreds of different ‘elementary’ particles formed by high energy collisions in particle accelerators using bubble chambers. These are large vessels of liquid hydrogen in a uniform magnetic field. Charged particles passing through the hydrogen leave a visible t ...
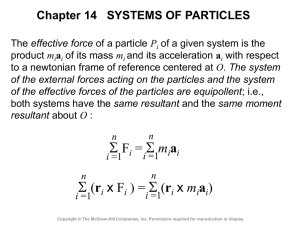
V - McGraw Hill Higher Education
... momentum is applied to a system S of particles during a time interval Dt, including particles which enter the system at A during that time interval and those (of the same mass Dm) which leave the system at B. The system formed by the momentum (Dm)vA of the particles entering S in the time Dt and the ...
... momentum is applied to a system S of particles during a time interval Dt, including particles which enter the system at A during that time interval and those (of the same mass Dm) which leave the system at B. The system formed by the momentum (Dm)vA of the particles entering S in the time Dt and the ...
Material since exam 3
... Question Inert gas atoms are ones that have just enough electrons to finish filling a p-shell (except for He). How many electrons do next two inert gas atoms after helium ( neon (Ne) and argon (Ar) ) have. In this range of atomic number the subshells fill in order of increasing angular momentum. ...
... Question Inert gas atoms are ones that have just enough electrons to finish filling a p-shell (except for He). How many electrons do next two inert gas atoms after helium ( neon (Ne) and argon (Ar) ) have. In this range of atomic number the subshells fill in order of increasing angular momentum. ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.