Material since exam 3
... Question Inert gas atoms are ones that have just enough electrons to finish filling a p-shell (except for He). How many electrons do next two inert gas atoms after helium ( neon (Ne) and argon (Ar) ) have. In this range of atomic number the subshells fill in order of increasing angular momentum. ...
... Question Inert gas atoms are ones that have just enough electrons to finish filling a p-shell (except for He). How many electrons do next two inert gas atoms after helium ( neon (Ne) and argon (Ar) ) have. In this range of atomic number the subshells fill in order of increasing angular momentum. ...
Conceptual Model for Diffusion
... model to predict the distribution of particle (mass) concentration , C(X,t). Note, that if we assume a unit mass per particle, we can conveniently interchange N = M. For simplicity we again consider a one-dimensional system, with the same rules of random motion described above, i.e. at each time ste ...
... model to predict the distribution of particle (mass) concentration , C(X,t). Note, that if we assume a unit mass per particle, we can conveniently interchange N = M. For simplicity we again consider a one-dimensional system, with the same rules of random motion described above, i.e. at each time ste ...
1 of 1 Atomic Structure Lingo Honors Freshman
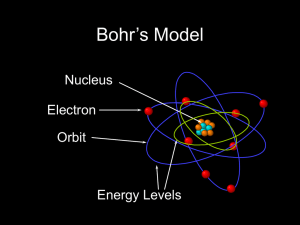
... nucleus - The center of an atom that has most of the mass of the atom. proton - A positively charged subatomic particle found in the nucleus of an atom. Mass is about 1 amu. atomic number - Represents the number of protons in the nucleus of an atom of an element. neutron - A neutral subatomic partic ...
... nucleus - The center of an atom that has most of the mass of the atom. proton - A positively charged subatomic particle found in the nucleus of an atom. Mass is about 1 amu. atomic number - Represents the number of protons in the nucleus of an atom of an element. neutron - A neutral subatomic partic ...
PowerPoint Presentation - Duality of Matter
... The wave nature makes it impossible to know with infinite precision how atomic matter moves. Specifically: To know a particle’s motion we must know its position and velocity at the same time. But how do you locate the position of a wave/particle electron? ...
... The wave nature makes it impossible to know with infinite precision how atomic matter moves. Specifically: To know a particle’s motion we must know its position and velocity at the same time. But how do you locate the position of a wave/particle electron? ...
Lab: Atoms and Eggs—Datasheet Name___________________
... Inside the nucleus of an atom are ______________ and _______________. The __________ ______________ of an atom is the sum of these two particles. The atomic number is equal to the number of __________________ present in the nucleus of the atom, and because the atom is neutral, the atomic number is a ...
... Inside the nucleus of an atom are ______________ and _______________. The __________ ______________ of an atom is the sum of these two particles. The atomic number is equal to the number of __________________ present in the nucleus of the atom, and because the atom is neutral, the atomic number is a ...
Midterm Exam No. 03 (Spring 2015) PHYS 520B: Electromagnetic Theory
... (b) Will a photon dispatched to ‘chase’ this particle at t = 0 from 0 < x < x0 ever catch up with it? If yes, when and where does it catch up? (c) Will a photon dispatched to ‘chase’ this particle, at t = 0 from x < x0 ever catch up with it? If yes, when and where does it catch up? 3. (30 points.) ...
... (b) Will a photon dispatched to ‘chase’ this particle at t = 0 from 0 < x < x0 ever catch up with it? If yes, when and where does it catch up? (c) Will a photon dispatched to ‘chase’ this particle, at t = 0 from x < x0 ever catch up with it? If yes, when and where does it catch up? 3. (30 points.) ...
nuclear review
... of nucleus, to make a 17) _____________ nucleus. This uses nuclear fusion of hydrogen atoms into helium atoms. This gives off heat and light and other radiation. When two types of hydrogen atoms, deuterium and tritium, combine to make a helium atom and an extra particle called a neutron plus energy, ...
... of nucleus, to make a 17) _____________ nucleus. This uses nuclear fusion of hydrogen atoms into helium atoms. This gives off heat and light and other radiation. When two types of hydrogen atoms, deuterium and tritium, combine to make a helium atom and an extra particle called a neutron plus energy, ...
L2 Atomic and Nuclear Physics 1
... – Democritus {Greece 460 B.C.} is credited with the idea that matter is made up of fundamental building blocks. He introduced the word atom to describe these building blocks. – In the 1930’s, the atom was believed to be constructed of neutrons, protons, and electrons. These were thought to have no s ...
... – Democritus {Greece 460 B.C.} is credited with the idea that matter is made up of fundamental building blocks. He introduced the word atom to describe these building blocks. – In the 1930’s, the atom was believed to be constructed of neutrons, protons, and electrons. These were thought to have no s ...
Science 9 Unit 2
... the reaction. E.g. a sugar cube takes longer to dissolve than regular refined sugar Energy – the type of energy used will determine how fast the reaction occurs. E.g. if you use electrical energy from a battery the reaction will be faster ...
... the reaction. E.g. a sugar cube takes longer to dissolve than regular refined sugar Energy – the type of energy used will determine how fast the reaction occurs. E.g. if you use electrical energy from a battery the reaction will be faster ...
May 2007
... be considered massless, interacting via the exchange of “gluons”, which are massless spin one bosons. At low temperatures the quarks and gluons don’t exist as free particles, they are confined inside the mesons. As the temperature rises, a transition takes place that frees the quarks and gluons. We ...
... be considered massless, interacting via the exchange of “gluons”, which are massless spin one bosons. At low temperatures the quarks and gluons don’t exist as free particles, they are confined inside the mesons. As the temperature rises, a transition takes place that frees the quarks and gluons. We ...
Basic Physics Concepts Useful in Astronomy
... If Object A exerts a force on Object B, then Object B must exert a force on Object A that is equal in magnitude, but opposite in direction Atoms o Everything in the Universe is made up of atoms o Atoms are made up of smaller particles o Atoms are held together by the positively charged nucleus and ...
... If Object A exerts a force on Object B, then Object B must exert a force on Object A that is equal in magnitude, but opposite in direction Atoms o Everything in the Universe is made up of atoms o Atoms are made up of smaller particles o Atoms are held together by the positively charged nucleus and ...
Wave
... – There is a tradeoff between particle and wave character. – Depending on the width of the wave packet, an object can be more like a particle or like a wave. – A particle (short wave packet) has a well-defined position, and a wave (long packet) has a well-defined momentum. ...
... – There is a tradeoff between particle and wave character. – Depending on the width of the wave packet, an object can be more like a particle or like a wave. – A particle (short wave packet) has a well-defined position, and a wave (long packet) has a well-defined momentum. ...
Condensed matter
... • Hydrogen storage—the energy problem • Astrophysics: dark matter and dark energy • Quantum computing, • Neutrino physics, • String theory • Particle physics, the Large Hadron Collider, the Higgs particle, Supersymmetric particles ...
... • Hydrogen storage—the energy problem • Astrophysics: dark matter and dark energy • Quantum computing, • Neutrino physics, • String theory • Particle physics, the Large Hadron Collider, the Higgs particle, Supersymmetric particles ...
Center of mass - Richard Barrans’s web site
... spring fires, one car moves off to the right and the other to the left. The speed of the car on the left is ...
... spring fires, one car moves off to the right and the other to the left. The speed of the car on the left is ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.