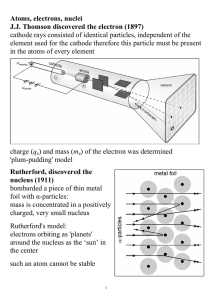
CHAPTER 5
... ---> s subshell with single suborbital For l = 1, ml = -1, 0, +1 ---> p subshell with 3 suborbitals For l = 2, ml = -2, -1, 0, +1, +2 ---> d subshell with 5 suborbitals For l = 3, ml = -3, -2, -1, 0, +1, +2, +3 ---> f subshell with 7 suborbitals ...
... ---> s subshell with single suborbital For l = 1, ml = -1, 0, +1 ---> p subshell with 3 suborbitals For l = 2, ml = -2, -1, 0, +1, +2 ---> d subshell with 5 suborbitals For l = 3, ml = -3, -2, -1, 0, +1, +2, +3 ---> f subshell with 7 suborbitals ...
Worksheet Key - UCSB C.L.A.S.
... a. It takes more energy to ionize the electron from n= 3 than from the ground state. b. The electron is farther from the nucleus on average in the n = 3 state than in the ground state c. The wavelength of light emitted if the electron drops from n = 3 to n = 2 is shorter than the wavelength of light ...
... a. It takes more energy to ionize the electron from n= 3 than from the ground state. b. The electron is farther from the nucleus on average in the n = 3 state than in the ground state c. The wavelength of light emitted if the electron drops from n = 3 to n = 2 is shorter than the wavelength of light ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... What are the eigen values of the parity operator? Show that the parity operator can have only two eigen values. 5. Express angular momentum operator 2 in terms of , where is position operator and is the momentum operator. ...
... What are the eigen values of the parity operator? Show that the parity operator can have only two eigen values. 5. Express angular momentum operator 2 in terms of , where is position operator and is the momentum operator. ...
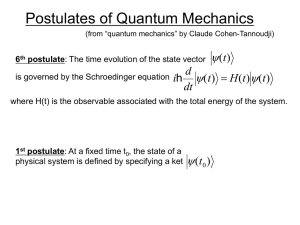
The World Of Quantum Mechanics
... under well-defined external conditions; that is to say, their paths are not as rigidly determined as at the classical level. This evolution is governed by laws of probability. In other words, while it is not possible to predict precisely the manner in which a given quantum entity will evolve under s ...
... under well-defined external conditions; that is to say, their paths are not as rigidly determined as at the classical level. This evolution is governed by laws of probability. In other words, while it is not possible to predict precisely the manner in which a given quantum entity will evolve under s ...
Relationship between the electric field and magnetic field
... We learned in the previous sections that E and B self-sustain each other (this is a consequence of the 3rd and 4th Maxwell's equation). We would expect, then, that in a harmonic EM-wave a direct relationship would exist between the electric field and magnetic field amplitudes. We show below that tha ...
... We learned in the previous sections that E and B self-sustain each other (this is a consequence of the 3rd and 4th Maxwell's equation). We would expect, then, that in a harmonic EM-wave a direct relationship would exist between the electric field and magnetic field amplitudes. We show below that tha ...
Pretest for Uncertainty Principle Part 1
... 2. Suppose both particles I and II are interacting with the same potential energy well. At time t=0, the wavefunction of particle I is ...
... 2. Suppose both particles I and II are interacting with the same potential energy well. At time t=0, the wavefunction of particle I is ...
Physics Final Review Sheet Name
... a. the length of the Great Wall of China c. the distance between two U.S Capitals b. the width of a locker d. the size of a virus 10.A person walks 2 miles every day for exercise, leaving her front porch at 9:00 A.M. and returning to her front porch at 9:45 A.M. What is the total displacement of her ...
... a. the length of the Great Wall of China c. the distance between two U.S Capitals b. the width of a locker d. the size of a virus 10.A person walks 2 miles every day for exercise, leaving her front porch at 9:00 A.M. and returning to her front porch at 9:45 A.M. What is the total displacement of her ...
2010 midterm exam solutions
... A beam of alpha particles is directed at a potential barrier as indicated in the figure below. The beam has a high flux of, Γ = 6 × 1021 particles/sec. The energy of the alpha particles is E = 5 MeV , and the potential barrier height is, VB = 85 MeV and its width is L = 10 fm. You may assume the alpha ...
... A beam of alpha particles is directed at a potential barrier as indicated in the figure below. The beam has a high flux of, Γ = 6 × 1021 particles/sec. The energy of the alpha particles is E = 5 MeV , and the potential barrier height is, VB = 85 MeV and its width is L = 10 fm. You may assume the alpha ...
File - Septor CORPORATION
... information processing with individual electrons and photons. S.K.Kurtz Prof Emeritus Electrical Engineering Penn State University ...
... information processing with individual electrons and photons. S.K.Kurtz Prof Emeritus Electrical Engineering Penn State University ...
From quantum to quantum computer
... (1) One of the founders of the quantum concept (2) A first, thought there must be something wrong with the quantum theory. (3) After much debate with Bohr, he finally was convinced that QM gives correct results, but it could not be the final theory. It is incomplete! ...
... (1) One of the founders of the quantum concept (2) A first, thought there must be something wrong with the quantum theory. (3) After much debate with Bohr, he finally was convinced that QM gives correct results, but it could not be the final theory. It is incomplete! ...
Problem Set 11
... the tip of a probe acts as a potential barrier to electrons bound to the specimen. A small bias between the specimen and the probe acts as a potential barrier of height V0 , and electrons can tunnel in this barrier to be detected at the probe as a small current. The tunnel current is very sensitive ...
... the tip of a probe acts as a potential barrier to electrons bound to the specimen. A small bias between the specimen and the probe acts as a potential barrier of height V0 , and electrons can tunnel in this barrier to be detected at the probe as a small current. The tunnel current is very sensitive ...
Uncertainty not so certain after all Early formulation
... Heisenberg’s original version still works for the light/electron example, Rozema says, but not in more general cases — as most scientists have assumed. In 2003, Japanese physicist Masanao Ozawa showed mathematically that Heisenberg’s first version couldn’t be right. Earlier this year, he and a resea ...
... Heisenberg’s original version still works for the light/electron example, Rozema says, but not in more general cases — as most scientists have assumed. In 2003, Japanese physicist Masanao Ozawa showed mathematically that Heisenberg’s first version couldn’t be right. Earlier this year, he and a resea ...
Accelerating Charge Through A Potential Difference
... Notice that energy given to the charged particle has no dependence at all on the distance d between the plates. It is only dependent on the charge of the particle and the potential difference between the plates ...
... Notice that energy given to the charged particle has no dependence at all on the distance d between the plates. It is only dependent on the charge of the particle and the potential difference between the plates ...
Quantum physics
... • Why rate of emission of electrons << rate of incidence of photons {for f>f0}: • Not every photon would collide with an electron; most are reflected by the metal or miss hitting any electron. • On the way out to the metal surface, an electron may lose its kinetic energy to ions and other electrons ...
... • Why rate of emission of electrons << rate of incidence of photons {for f>f0}: • Not every photon would collide with an electron; most are reflected by the metal or miss hitting any electron. • On the way out to the metal surface, an electron may lose its kinetic energy to ions and other electrons ...