lecture25
... If light is particles, theory predicts: • Increasing intensity increases number of electrons but not energy • Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency • There is a cutoff frequency below which no electrons will be emitted, regardless ...
... If light is particles, theory predicts: • Increasing intensity increases number of electrons but not energy • Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency • There is a cutoff frequency below which no electrons will be emitted, regardless ...
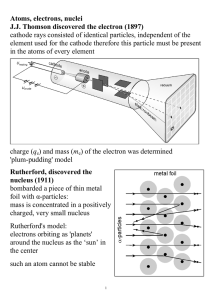
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... de Broglie (1923) described the discrete energy levels of electrons within an atom as results of a wave phenomenon momentum of an electron p = mev = h/p where λ is the wavelength of the matter wave corresponding to the electron, and h is the Planck constant. Davisson and Germer (1927) used electro ...
... de Broglie (1923) described the discrete energy levels of electrons within an atom as results of a wave phenomenon momentum of an electron p = mev = h/p where λ is the wavelength of the matter wave corresponding to the electron, and h is the Planck constant. Davisson and Germer (1927) used electro ...
CHM1045 - Michael Blaber
... En = -RH / n2 or En = -B / n2 (the relationship between the energy of an electron in Bohr's model of the hydrogen atom, and the orbit number of the electron) Elevel = RH * (1/ni2 - 1/nf2) or En = B * (1/ni2 - 1/nf2) (Energy change associated with an electron transition from one orbit to another in ...
... En = -RH / n2 or En = -B / n2 (the relationship between the energy of an electron in Bohr's model of the hydrogen atom, and the orbit number of the electron) Elevel = RH * (1/ni2 - 1/nf2) or En = B * (1/ni2 - 1/nf2) (Energy change associated with an electron transition from one orbit to another in ...
Document
... of ejected electrons is proportional to intensity Electrons are ejected instantly, regardless of intensity level For constant intensity, the # of electrons decreases with increasing frequency If the frequency is below a certain level, no electrons are ejected, regardless of intensity ...
... of ejected electrons is proportional to intensity Electrons are ejected instantly, regardless of intensity level For constant intensity, the # of electrons decreases with increasing frequency If the frequency is below a certain level, no electrons are ejected, regardless of intensity ...
Chapter 7 Quantum Theory of the Atom
... Experiments so far demonstrated: 1) e– are ejected from the surface only if light of a minimum frequency is used. 2) The number of e– ejected is directly proportional to the intensity of light. 3) Wave theory alone could not explain this phenomenon. ...
... Experiments so far demonstrated: 1) e– are ejected from the surface only if light of a minimum frequency is used. 2) The number of e– ejected is directly proportional to the intensity of light. 3) Wave theory alone could not explain this phenomenon. ...
Supplement to Science Club reading for
... less precise another measurement pertaining to the same particle (such as its momentum) must become. In 1905, Albert Einstein suggested that not just matter but also energy could be “quantized”: the energ ...
... less precise another measurement pertaining to the same particle (such as its momentum) must become. In 1905, Albert Einstein suggested that not just matter but also energy could be “quantized”: the energ ...
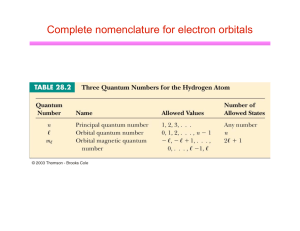
Complete nomenclature for electron orbitals
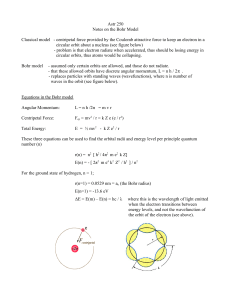
... that angular momentum of the hydrogen atom is quantized: mevr = nh • Why? Not known for 10 years until de Broglie gave a physical interpretation. • Electron orbit could be stable only if an integral number of electron wavelengths could be fit inside orbit. • 2pr = nl n=1,2,3,… ...
... that angular momentum of the hydrogen atom is quantized: mevr = nh • Why? Not known for 10 years until de Broglie gave a physical interpretation. • Electron orbit could be stable only if an integral number of electron wavelengths could be fit inside orbit. • 2pr = nl n=1,2,3,… ...
Ch # 17 Advent of Modern Physics Special Theory Of Relativity
... 1. The rest mass of photon is __________. (One, Zero, Infinite, None of these) 2. In 1905, __________ proposed that the packets or bundles of energy are integral part of all electromagnetic radiations. (Plank, Einstein, Newton, Wein) 3. The process of ejection of loosely bound electrons from a metal ...
... 1. The rest mass of photon is __________. (One, Zero, Infinite, None of these) 2. In 1905, __________ proposed that the packets or bundles of energy are integral part of all electromagnetic radiations. (Plank, Einstein, Newton, Wein) 3. The process of ejection of loosely bound electrons from a metal ...
Slide 1
... E-M radiation was considered to be a wave/energy phenomenon and not matter Max Planck developed a new physics when classical physics could not be used to interpret data from blackbody radiation ( Blackbody is an object that absorbs all radiation incident on it) Blackbody radiation is emitted by sol ...
... E-M radiation was considered to be a wave/energy phenomenon and not matter Max Planck developed a new physics when classical physics could not be used to interpret data from blackbody radiation ( Blackbody is an object that absorbs all radiation incident on it) Blackbody radiation is emitted by sol ...
Interference of Waves
... Light behaves as a wave sometime, and behaves like a particle in other cases, as is called particle-wave duality. ...
... Light behaves as a wave sometime, and behaves like a particle in other cases, as is called particle-wave duality. ...
1-d examples
... In the above example, the particle was able to penetrate into the classically disallowed region, though not very effectively, since the wavefunction decayed away exponentially the further you went into that region. But what if the classicaly forbidden region has only finite thickness? ...
... In the above example, the particle was able to penetrate into the classically disallowed region, though not very effectively, since the wavefunction decayed away exponentially the further you went into that region. But what if the classicaly forbidden region has only finite thickness? ...
Chapter 7
... When the particle is not at the boundaries it feels no potential (V=0) so it acts like a free particle. We saw that the free particles had a definite wavelength, λ. In addition, at the boundaries \ it is impossible for the particle to enter the forbidden \ region, therefore the wave function must go ...
... When the particle is not at the boundaries it feels no potential (V=0) so it acts like a free particle. We saw that the free particles had a definite wavelength, λ. In addition, at the boundaries \ it is impossible for the particle to enter the forbidden \ region, therefore the wave function must go ...